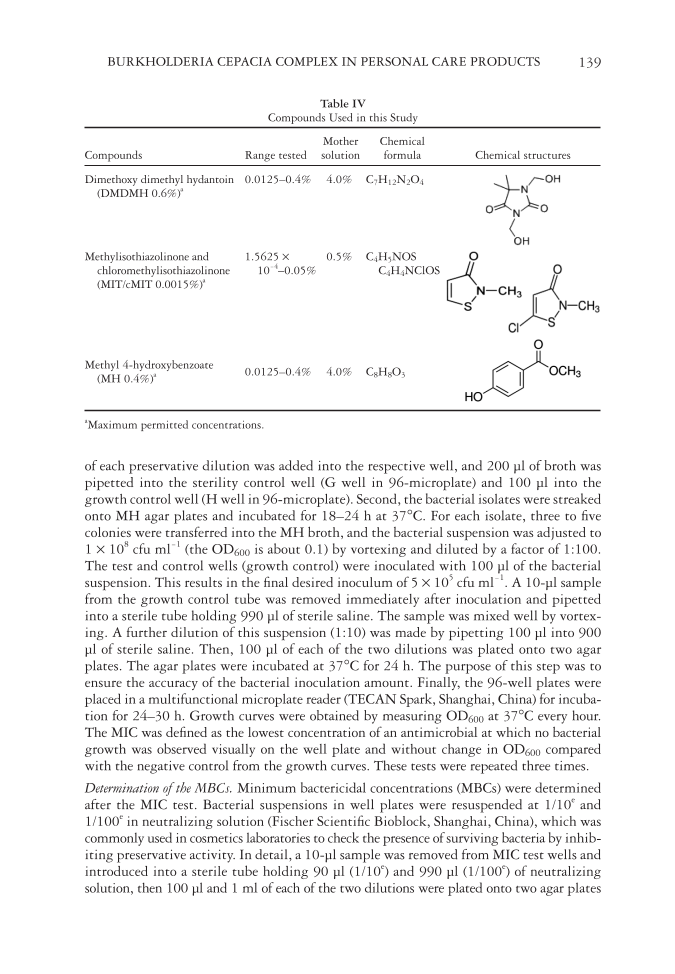
BURKHOLDERIA CEPACIA COMPLEX IN PERSONAL CARE PRODUCTS 139 Table IV Compounds Used in this Study Compounds Range tested Mother solution Chemical formula Chemical structures Dimethoxy dimethyl hydantoin (DMDMH 0.6%)a 0.0125–0.4% 4.0% C7H12N2O4 Methylisothiazolinone and chloromethylisothiazolinone (MIT/cMIT 0.0015%)a 1.5625 × 10-4–0.05% 0.5% C4H5NOS C4H4NClOS Methyl 4-hydroxybenzoate (MH 0.4%)a 0.0125–0.4% 4.0% C8H8O3 a Maximum permitted concentrations. of each preservative dilution was added into the respective well, and 200 μl of broth was pipetted into the sterility control well (G well in 96-microplate) and 100 μl into the growth control well (H well in 96-microplate). Second, the bacterial isolates were streaked onto MH agar plates and incubated for 18–24 h at 37°C. For each isolate, three to fi ve colonies were transferred into the MH broth, and the bacterial suspension was adjusted to 1 × 108 cfu ml-1 (the OD600 is about 0.1) by vortexing and diluted by a factor of 1:100. The test and control wells (growth control) were inoculated with 100 μl of the bacterial suspension. This results in the fi nal desired inoculum of 5 × 105 cfu ml-1. A 10-μl sample from the growth control tube was removed immediately after inoculation and pipetted into a sterile tube holding 990 μl of sterile saline. The sample was mixed well by vortex- ing. A further dilution of this suspension (1:10) was made by pipetting 100 μl into 900 μl of sterile saline. Then, 100 μl of each of the two dilutions was plated onto two agar plates. The agar plates were incubated at 37°C for 24 h. The purpose of this step was to ensure the accuracy of the bacterial inoculation amount. Finally, the 96-well plates were placed in a multifunctional microplate reader (TECAN Spark, Shanghai, China) for incuba- tion for 24–30 h. Growth curves were obtained by measuring OD600 at 37°C every hour. The MIC was defi ned as the lowest concentration of an antimicrobial at which no bacterial growth was observed visually on the well plate and without change in OD600 compared with the negative control from the growth curves. These tests were repeated three times. Determination of the MBCs. Minimum bactericidal conce ntrations (MBCs) were dete rmined after the MIC test. Bacterial suspensions in well plates were resuspended at 1/10e and 1/100e in neutralizing solution (Fischer Scientifi c Bioblock, Shanghai, China), which was commonly used in cosmetics laboratories to check the presence of surviving bacteria by inhib- iting preservative activity. In detail, a 10-μl sample was removed from MIC test wells and introduced into a sterile tube holding 90 μl (1/10e) and 990 μl (1/100e) of neutralizing solution, then 100 μl and 1 ml of each of the two dilutions were plated onto two agar plates
JOURNAL OF COSMETIC SCIENCE 140 and incubated at 37°C for 24 h (40). The MBC was estimated as the lowest concentration of preservative with the absence of colony in the case of agar plates. RESULTS BCC SPECIES ISOLATION AND IDENTIFICATION We collec ted 25 B cc strains from 2015 to 2017, isolated fr om shampoos, hair conditioners, raw materials, body lotion, shower gel, toner, cream, production water, and bubble water. The number of bacteria exceeds 103 cfu ml-1, of which 18 Bcc strains are greater than 105 cfu ml-1. The types of preservatives were MIT/cMIT, DMDMH, and MH. Except for the two PCPs (body lotion and shower gel) collected in July and November 2016, other types of cosmetics can be detected with residual MIT, the content of which is between 10.8 and 15.6 mg kg-1 (Table II). All of the spoilage strains were Gram-negative, non–spore-forming rods, and four species were identifi ed by recA nucleotide sequence analysis, including Burkholderia cenocepacia, B. contaminans, Burkholderia lata, and B. cepacia, with the phylo- genetic tree of Bcc based on the recA gene sequence (Figure 1). BCC SPECIES DIVERSITY IN CONTAMINATED PCPS RecA gene sequence and MLST analysis of a collection of 25 Bcc is olates recovered from PCPs revealed the following species diversity: B. cenocepacia (ST621, ST258, and nST), B. lata (ST339 and ST336), B. contaminans (ST482), and B. cepacia (ST922). A total of seven distinct STs were identifi ed belonging to four different Bcc species, including one novel ST. The new ST type, BC-04, was identifi ed as B. cenocepacia by recA gene, the evolution- ary tree established by recA gene showed that it was not in the same branch as other B. cenocepacia, and the MLST results also showed the gltB and gyrB were 64 and 506, which were different among the seven housekeeping genes. These all indicate that BC-04 might be the new ST type of B. cenocepacia (Figure 1). The proportion of 48% of stains we de- tected is B. cenocepacia ST621, the dominant STs were ST621 among all ST types, fol- lowed by ST339 (16%) and ST336 (16%). The colony morphology of Bcc species grown on agar was different according to STs (Fig- ure 2). Different ST types in the same Bcc show different colony morphologies. It was worth noting that the colonies of Burkholderia cepacia (ST922) showed a purple pigment spreading from the center to the edge (add 5 μl of the bacterial solution that was stored in a glycerol storage tube to the TSA plate and incubate at 37°C for 5 days) this phenom- enon might become a specifi c phenotype of ST922. SUSCEPTIBILITY OF BCC STRAINS ISOLATED FROM CONTAMINATED PCPS MIC a nd MBC methods allow the rapid evidence of bacterial susceptib ility in response to biocides contact and action in general, and to preservatives in particular. In this study, the methods were used to evaluate the susceptibility of preservatives agents (DMDMH, MIT/ cMIT, and MH) for a collection of 25 Bcc strains and B. cepacia ATCC 25416. The mean of MICs and MBCs demonstrated susceptibility varied within Bcc species (Table V). Our
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)