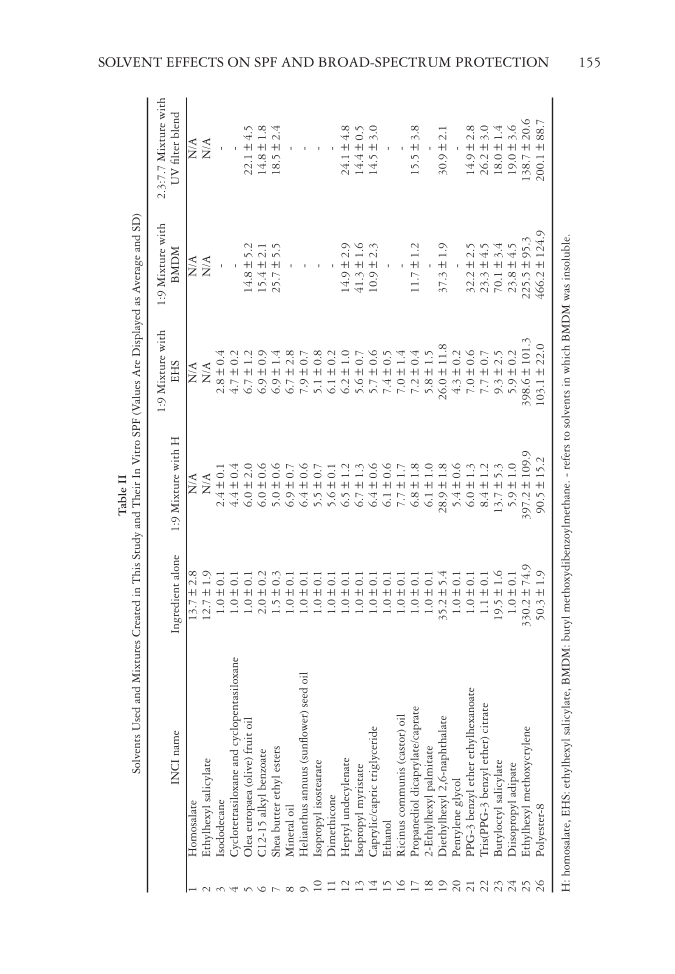
Table II Solvents Used and Mixtures Created in This Study and Their In Vitro SPF (Values Are Displayed as Average and SD) INCI name Ingredient alone 1:9 Mixture with H 1:9 Mixture with EHS 1:9 Mixture with BMDM 2.3:7.7 Mixture with UV fi lter blend 1 Homosalate 13.7 ± 2.8 N/A N/A N/A N/A 2 Ethylhexyl salicylate 12.7 ± 1.9 N/A N/A N/A N/A 3 Isododecane 1.0 ± 0.1 2.4 ± 0.1 2.8 ± 0.4 - - 4 Cyclotetrasiloxane and cyclopentasiloxane 1.0 ± 0.1 4.4 ± 0.4 4.7 ± 0.2 - - 5 Olea europaea (olive) fruit oil 1.0 ± 0.1 6.0 ± 2.0 6.7 ± 1.2 14.8 ± 5.2 22.1 ± 4.5 6 C12-15 alkyl benzoate 2.0 ± 0.2 6.0 ± 0.6 6.9 ± 0.9 15.4 ± 2.1 14.8 ± 1.8 7 Shea butter ethyl esters 1.5 ± 0.3 5.0 ± 0.6 6.9 ± 1.4 25.7 ± 5.5 18.5 ± 2.4 8 Mineral oil 1.0 ± 0.1 6.9 ± 0.7 6.7 ± 2.8 - - 9 Helianthus annuus (sunfl ower) seed oil 1.0 ± 0.1 6.4 ± 0.6 7.9 ± 0.7 - - 10 Isopropyl isostearate 1.0 ± 0.1 5.5 ± 0.7 5.1 ± 0.8 - - 11 Dimethicone 1.0 ± 0.1 5.6 ± 0.1 6.1 ± 0.2 - - 12 Heptyl undecylenate 1.0 ± 0.1 6.5 ± 1.2 6.2 ± 1.0 14.9 ± 2.9 24.1 ± 4.8 13 Isopropyl myristate 1.0 ± 0.1 6.7 ± 1.3 5.6 ± 0.7 41.3 ± 1.6 14.4 ± 0.5 14 Caprylic/capric triglyceride 1.0 ± 0.1 6.4 ± 0.6 5.7 ± 0.6 10.9 ± 2.3 14.5 ± 3.0 15 Ethanol 1.0 ± 0.1 6.1 ± 0.6 7.4 ± 0.5 - - 16 Ricinus communis (castor) oil 1.0 ± 0.1 7.7 ± 1.7 7.0 ± 1.4 - - 17 Propanediol dicaprylate/caprate 1.0 ± 0.1 6.8 ± 1.8 7.2 ± 0.4 11.7 ± 1.2 15.5 ± 3.8 18 2-Ethylhexyl palmitate 1.0 ± 0.1 6.1 ± 1.0 5.8 ± 1.5 - - 19 Diethylhexyl 2,6-naphthalate 35.2 ± 5.4 28.9 ± 1.8 26.0 ± 11.8 37.3 ± 1.9 30.9 ± 2.1 20 Pentylene glycol 1.0 ± 0.1 5.4 ± 0.6 4.3 ± 0.2 - - 21 PPG-3 benzyl ether ethylhexanoate 1.0 ± 0.1 6.0 ± 1.3 7.0 ± 0.6 32.2 ± 2.5 14.9 ± 2.8 22 Tris(PPG-3 benzyl ether) citrate 1.1 ± 0.1 8.4 ± 1.2 7.7 ± 0.7 23.3 ± 4.5 26.2 ± 3.0 23 Butyloctyl salicylate 19.5 ± 1.6 13.7 ± 5.3 9.3 ± 2.5 70.1 ± 3.4 18.0 ± 1.4 24 Diisopropyl adipate 1.0 ± 0.1 5.9 ± 1.0 5.9 ± 0.2 23.8 ± 4.5 19.0 ± 3.6 25 Ethylhexyl methoxycrylene 330.2 ± 74.9 397.2 ± 109.9 398.6 ± 101.3 225.5 ± 95.3 138.7 ± 20.6 26 Polyester-8 50.3 ± 1.9 90.5 ± 15.2 103.1 ± 22.0 466.2 ± 124.9 200.1 ± 88.7 H: homosalate, EHS: ethylhexyl salicylate, BMDM: butyl methoxydibenzoylmethane. - refers to solvents in which BMDM was insoluble. SOLVENT EFFECTS ON SPF AND BROAD-SPECTRUM PROTECTION 155
JOURNAL OF COSMETIC SCIENCE 156 The four solvents that had the highe st SPF alone shared common structural characteristics, including ester bonds, conjugated structure, and aromatic ring(s). Aromatic rings and a conjugated structure potentially allowed for many possible resonance structures to exist when excited with an electron. Stronger absorption at longer wavelengths equates to a higher SPF value. Aromatic rings have an utmost importance for the UV spectroscopic properties of molecules. Nothing proves its importance more than the fact that today all organic UV fi lters have aromatic moieties (22). In addition, ethylhexyl methoxycrylene and polyester-8 also had cyano (–CN) groups in their conjugated structure. CN groups have two pi (π) bonds, which allow for more electron delocalization compared with a double bond that has just one π bond. When counting the total number of π bonds pres- ent in each molecule, polyester-8 has 22, ethylhexyl methoxycrylene has 10, diethylhexyl 2,6-naphthalate has seven, and tris(PPG-3 benzyl ether) citrate has 12. However, it is also important to understand that the most stable molecules have their π bonds in a conju- gated structure. Thus, although tris(PPG-3 benzyl ether) citrate has 12 π bonds overall, only three are positioned in a conjugated manner. In comparison, diethylhexyl 2,6-naph- thalate only has seven π bonds overall however, all are in a conjugated structure together. This is why diethylhexyl 2,6-naphthalate had a higher SPF in every case. When looking at ethylhexyl methoxycrylene, it has all of its 10 π bonds in a conjugated structure, which explains its higher SPF. Although these structural characteristics ar e important, their presence did not guarantee a solvent to have a high SPF alone. For example, C12-15 alkyl benzoate, PPG-3 benzyl ether ethylhexanoate, and tris(PPG-3 benzyl ether) citrate had aromatic rings and conju- gated structure however, their in vitro SPF alone was around 1. Some solvents, such as Olea europaea (olive) oil, shea butter ethyl esters, heptyl undecylenate, and diisopropyl adipate contained ester bonds but did not possess aromatic moieties or a conjugated structure. The in vitro SPF of these solvents was also around 1. They all signifi cantly boosted the SPF when combined with UV fi lters ( 0.05), especially tris(PPG-3 benzyl ether) citrate, which achieved one of the highest SPF values for the UV fi lter blend, but they did not have a high SPF themselves. In addition to strictly looking at the chemi cal structure, the mechanism of action of the solvents also has to be considered. Some solvents tested in this study are sold as photosta- bilizers. When chemical UV fi lters absorb light, the energy from the UV photons convert them from the ground state to the excited state. After the energy is dissipated, electrons will return to the ground state, and the UV fi lter is ready to receive the next UV photon. Some UV fi lters, such as butyl methoxydibenzoylmethane, are photounstable, and their chemical structure changes in the excited state, which prevents them from absorbing the next UV photon. Photostabilizers are molecules that are able to reduce or avoid the pho- todegradation of UV fi lters. A common approach to photostabilization is to quench the excited state (either singlet or triplet state) of the UV fi lter and quickly return the UV fi lter to the ground state (23,24). Examples for this mechanism include triplet-state quenchers diethylhexyl 2,6-naphthalate and polyester-8 and singlet-state quencher eth- ylhexyl methoxycrylene. For a photostabilizer to be effective, it has to show a similar energy level to that of the photoexcited state of the photounstable molecule to absorb the excitation energy (7). This is the reason why photostabilizers are often also UV absorbers (25), as we also observed this phenomenon in this work. The photostabilizing mechanism of action of butyloctyl salicylate is different from the aforementioned solvents. Butyloctyl salicylate has a high dielectric constant, making it highly polar. Matching the polarity of
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)