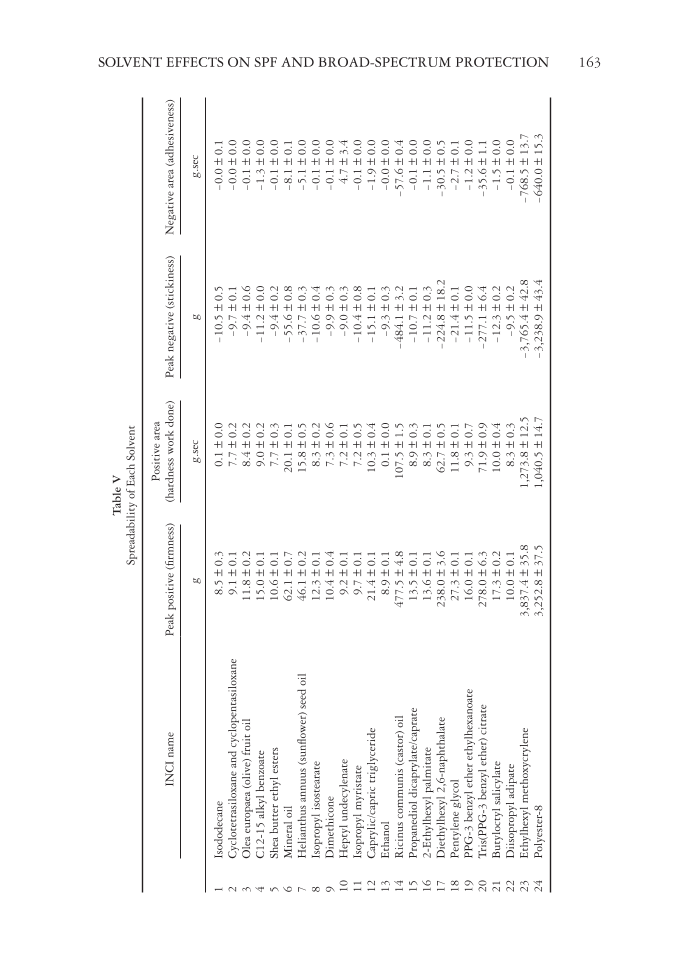
Table V Spreadability of Each Solvent INCI name Peak positive (fi rmness) Positive area (hardness work done) Peak negative (stickiness) Negative area (adhesiveness) g g.sec g g.sec 1 Isododecane 8.5 ± 0.3 0.1 ± 0.0 -10.5 ± 0.5 -0.0 ± 0.1 2 Cyclotetrasiloxane and cyclopentasiloxane 9.1 ± 0.1 7.7 ± 0.2 -9.7 ± 0.1 -0.0 ± 0.0 3 Olea europaea (olive) fruit oil 11.8 ± 0.2 8.4 ± 0.2 -9.4 ± 0.6 -0.1 ± 0.0 4 C12-15 alkyl benzoate 15.0 ± 0.1 9.0 ± 0.2 -11.2 ± 0.0 -1.3 ± 0.0 5 Shea butter ethyl esters 10.6 ± 0.1 7.7 ± 0.3 -9.4 ± 0.2 -0.1 ± 0.0 6 Mineral oil 62.1 ± 0.7 20.1 ± 0.1 -55.6 ± 0.8 -8.1 ± 0.1 7 Helianthus annuus (sunfl ower) seed oil 46.1 ± 0.2 15.8 ± 0.5 -37.7 ± 0.3 -5.1 ± 0.0 8 Isopropyl isostearate 12.3 ± 0.1 8.3 ± 0.2 -10.6 ± 0.4 -0.1 ± 0.0 9 Dimethicone 10.4 ± 0.4 7.3 ± 0.6 -9.9 ± 0.3 -0.1 ± 0.0 10 Heptyl undecylenate 9.2 ± 0.1 7.2 ± 0.1 -9.0 ± 0.3 4.7 ± 3.4 11 Isopropyl myristate 9.7 ± 0.1 7.2 ± 0.5 -10.4 ± 0.8 -0.1 ± 0.0 12 Caprylic/capric triglyceride 21.4 ± 0.1 10.3 ± 0.4 -15.1 ± 0.1 -1.9 ± 0.0 13 Ethanol 8.9 ± 0.1 0.1 ± 0.0 -9.3 ± 0.3 -0.0 ± 0.0 14 Ricinus communis (castor) oil 477.5 ± 4.8 107.5 ± 1.5 -484.1 ± 3.2 -57.6 ± 0.4 15 Propanediol dicaprylate/caprate 13.5 ± 0.1 8.9 ± 0.3 -10.7 ± 0.1 -0.1 ± 0.0 16 2-Ethylhexyl palmitate 13.6 ± 0.1 8.3 ± 0.1 -11.2 ± 0.3 -1.1 ± 0.0 17 Diethylhexyl 2,6-naphthalate 238.0 ± 3.6 62.7 ± 0.5 -224.8 ± 18.2 -30.5 ± 0.5 18 Pentylene glycol 27.3 ± 0.1 11.8 ± 0.1 -21.4 ± 0.1 -2.7 ± 0.1 19 PPG-3 benzyl ether ethylhexanoate 16.0 ± 0.1 9.3 ± 0.7 -11.5 ± 0.0 -1.2 ± 0.0 20 Tris(PPG-3 benzyl ether) citrate 278.0 ± 6.3 71.9 ± 0.9 -277.1 ± 6.4 -35.6 ± 1.1 21 Butyloctyl salicylate 17.3 ± 0.2 10.0 ± 0.4 -12.3 ± 0.2 -1.5 ± 0.0 22 Diisopropyl adipate 10.0 ± 0.1 8.3 ± 0.3 -9.5 ± 0.2 -0.1 ± 0.0 23 Ethylhexyl methoxycrylene 3,837.4 ± 35.8 1,273.8 ± 12.5 -3,765.4 ± 42.8 -768.5 ± 13.7 24 Polyester-8 3,252.8 ± 37.5 1,040.5 ± 14.7 -3,238.9 ± 43.4 -640.0 ± 15.3 SOLVENT EFFECTS ON SPF AND BROAD-SPECTRUM PROTECTION 163
JOURNAL OF COSMETIC SCIENCE 164 structural elements, including ester bonds, conjugated structure, and aromatic ring(s). Ethylhexyl methoxycrylene and polyester-8 also had –CN groups. These four solvents had an inherent SPF-absorbing capacity as well, which led to the high SPF of the UV fi lter–solvent mixtures. Our results indicated that these structural characteristics are important and can in- dicate good performance however, the absence of some of these structural elements did not necessarily prevent a solvent from being a booster. Multiple solvents caused a signifi cant hyp- sochromic or bathochromic shift in the UV fi lters’ λmax, especially for butyl methoxydibenzo- ylmethane. However, in all cases, the λmax stayed in the range where the UV fi lter was normally absorbed in. In the case of the UVB fi lters, most solvents only caused a minimal or no shift. Although polyester-8 and ethylhexyl methoxycrylene achieved the highest SPF values, they were viscous and sticky and had a strong color. Optimizing their use level is recommended to achieve good performance and acceptable aesthetics. For a formulator t o select and estimate the effect of a solvent on the UV-absorbing char- acteristics of a UV fi lter, solubility of the UV fi lter in the solvent, chemical structure, and mechanism of action of the solvent and solvent–UV fi lter interactions should be consid- ered. Results of this study provide practical information that can guide sunscreen formu- lators in selecting solvents for UV fi lters and making more effective sunscreens. ACKNOWLEDGMENTS We would like to e xpress our thanks to the suppliers, including Symrise, Croda, BASF, East- man, Active Concepts, Innospec, AAK, Lubrizol, INOLEX, Hallstar, and Wacker Chemie for providing us with samples of their ingredients. We also acknowledge the assistance of Katie Wolf, Jess Kona-Stanciu, and Janelle Barth in this project. We acknowledge the partial sup- port from Access Business Group International LLC under award number N-127059-01. REFERENCES (1) H. H önigsmann, E rythema and pigmentation, Photodermatol. Photoimmunol. Photomed., 18, 75–81 (2002). (2) J. H. Rabe, A. J . Mamelak, P. J. McElgunn, W. L. Morison, and D. N. Sauder, Photoaging: mechanisms and repair, J. Am. Acad. Dermatol., 55, 1–19 (2006). (3) Hawaii State Senate, A bill for an act relating to water pollution, 29th Legislature SB 2571, 2018, ac- cessed December 4, 2019, https://www.capitol.hawaii.gov/session2018/bills/SB2571_.HTM. (4) City of Key West, FL City Commission, Action Minutes—Final, 2019, accessed December 4, 2019, http://keywestcity.granicus.com/MediaPlayer.php?view_id=1&clip_id=941. (5) Committee on Government Operations, Consumers, and Veterans Affair, Thirty-Third Legislature of the Virgin Islands, 2019, accessed December 4, 2019, http://legvi.org/committeemeetings/Rules%20 and%20Judiciary/Thursday,%20June%2013,%202019/Bills/33-0043.pdf (6) S. L. Schneider a nd H. W. Lim, Review of environmental effects of oxybenzone and other sunscreen ac- tive ingredients, J. Am. Acad. Dermatol., 80, 266–271 (2019). (7) S. Q. Wang and H . W. Lim, Principles and Practice of Photoprotection (Springer International Publish- ing, Cham, Switzerland, 2016). (8) S. Abbott, Solubi lity Science: Principles and Practice (Creative Commons BY-ND, Attribution and No- Derivatives, 2018), https://www.stevenabbott.co.uk/_downloads/Solubility%20Science%20Principles%20 and%20Practice.pdf. (9) J. Wiechers and S . J. Abbott, Formulating for Effi cacy, the Software, 2011/2013, accessed August 24, 2019, https://www.jwsolutionssoftware.com/. (10) M. A. Kalam, S. Alsheri, A. Alshamsan, M. Alkholief, R. Ali, and F. Shakeel, Solubility measurement, Hansen solubility parameters and solution thermodynamics of gemfi brozil in different pharmaceutically used solvents, Drug Dev. Ind. Pharm., 45, 1258–1264 (2019).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)