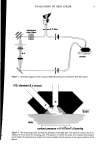
EVALUATION OF NAIL ENAMEL 51 glycol and then stirred vigorously with a magnetic mixer. The Karl Fischer reagent, which contains iodine, sulfur dioxide, pyridine and methanol, is slowly added from a burette. A series of chemical reactions involving any water present takes place. When all water has been consumed a brown color developes due to the presence of excess iodine. This same end point may be obtained more precisely by an electrical method. Either way, the water content may be calculated from the amounts of the sample and reagent used. WATER AND DETERGENT RESISTANCE A water immersion test is used to determine water resistance of a lacquer. A 0.0006-in. film on three glass plates should be applied and dried in an oven at 25øC for 24 h. The plates should be removed and placed in a dessicator for another 24 h and then removed and weighed to the nearest 0.10 rag. The plates should then be immersed in a water bath containing distilled water at 37øC for 24 h. The panels should then be removed and dried by placing the plate between absorption paper and reweighed. They are again accurately weighed and the loss in weight computed as percent of the original weight of the sample. Detergent resistance may be determined by ASTM D 1647 by the following method: A specimen of each enamel is prepared by dipping a test tube in the lacquer. The test tubes are inverted and supported on vertical pegs during the drying period (72 h). A 3 percent Tide solution in water is prepared. The specimens are dipped into the solution without touching the wall or bottom of the beaker. After 72 h the specimens are removed, rinsed under a gentle stream of water, allowed to dry for 30 rain and examined for whittening, blistering or removal of film. The test may be continued for a week (168 h), if necessary. Finally, toxicity tests may be administered on nail enamels in accordance with the Federal Hazardous Substances Act Regulations. Acute Oral Toxicity (Rat), Primary Derreal Irritation (Rabbit), Acute Dermal Toxicity (Rabbit), Skin Sensitization (Guinea Pig), and Draize Ocular Irritation (Rabbit) are common for nail enamels. Human use tests involving 50-100 subjects may also be employed to determine if the product is an irritant or sensitizer. CONCLUSIONS Nail enamels should be easy to apply and should dry and harden rapidly. They should be waterproof, well adherent, glossy, elastic, resistant to chipping and abrasion. Lastly, lacquers must be non-toxic, non-irritating and non-sensitizing to the skin. The behavior of nail enamel on a substrate provides a good indication of its behavior on finger nails but it is still necessary to study the product by actual application to the nails. Again tests are made on parameters such as flow, evenness of application, drag on the applicator brush, smoothness of the dried film, hardness, gloss and wear resistance. Ultimately, all of the aforementioned must be evaluated on nails. Nail enamels must be carefully tested to make sure that they meet the performance specifications which have been set up by both marketing and technical executives. The judicial selection of testing methods and the correct interpretation of data are vitally
52 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS important to the development of improved nail coating formulations. The perfor- mance of a nail enamel and its components is of primary concern to the lacquer chemist, he must prescribe the tests, interpret and redefine the data while recognizing their overall significance, and apply conclusions based on the information obtained in the tests. REFERENCES (1) Peter Diamandis, Eye, Lip and Nail Care, Product Marketing, p. S60-62 (Special Issue, Winter 1980). (2) H.J. Wing in The Chemistry And Manufacture Of Cosmetics, Volume IV, 2nd ed., M. G. deNavarre, Ed., (Continental Press, Orlando, FL, 1975), pp 1001-1002. (3) A. Shansky, Establishing physical parameters of nail lacquers, Drug & Cosmetics Industries, 123 (5), 48 (1978). ß (4) M. Schlossman, Modern nail enamel technology, J. Soc. Cosmet. Chem., 31, 29-36 (1980). (5) F. W. Busch, Jr. and M. G. Brookins, "Thixotropic Flow in Cosmetics: Utility and Evaluation," presented at the New England Chapter of the Society of Cosmetic Chemists, Boston, April 25, 1974.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)