UV PROTECTION AND EVALUATION OF EFFICACY OF SUNSCREENS 319 Table I Comparison of Structural and Functional Changes in Skin during Intrinsic and Photoaging Particular Photoaging Intrinsic aging Epidermal changes Thickness increases, acanthropic in early phase and atrophy in later stages Thin epidermis Proliferative rate is higher than normal Proliferative rate is lower than normal Non-uniform and random distribution of keratinocytes, polarity of cells is lost, frequent enlargement Uniform and defi ned distribution of keratinocytes, polarity is maintained, usually atrophied Diversifi ed melanosomes Uniformly distributed melanosomes Increased number of stratum corneum (SC) cell layer Normal cell layer Vitamin A content is destroyed by sun exposure Plasma content of retinol increases Dermal changes Marked elastogenesis followed by massive degeneration Elastogenesis followed by elastolysis Massive increase in elastic fibers Gradual decline in production of dermal matrix Increased lysozyme deposition on elastic fibers Modest lysozyme deposition on elastic fibers Decrease in amounts of mature collagen Mature collagen more stable in degradation Increased mast cells Decreased mast cells Vessels become dilated Microvessels decrease Pronounced inflammation No inflammatory response Marked increase in glycosaminoglycans Slight decrease in glycosaminoglycans Common signs and symptoms Mild (age 28–35 years): Few wrinkles, no keratoses Fine wrinkles, thin and transparent skin Moderate (age 35–50 years): Early wrinkling, sallow complexion with early actinic keratoses Loss of underlying fat leading to hollowed cheeks and eye sockets with noticeable loss of fi rmness on the hands and neck Advanced (age 50–60 years): Persistent wrinkling, discoloration of the skin with telangiectases and actinic keratoses Bones shrink away from the skin as a result of bone loss, which causes sagging of skin, dry skin with pruritus Severe (age 65–70 years): Severe wrinkling, photo aging, gravitational and dynamic forces affecting the skin, actinic keratoses with or without skin cancer Inability to sweat suffi ciently to cool the skin, faster graying of hair these cells escape programmed cell death (10). On absorption of UVB by DNA, photo- products (thymine dimers) are formed that lead to UV mutations (23). Signaling pathway involved in the formation of sunburn cells is explained in Figure 5. MECHANISM OF IMMUNOSUPPRESSION UV exposure suppresses immune responses, like contact hypersensitivity (CHS) reactions to chemical haptens (24), delayed-type hypersensitivity (DTH) reactions toward viral (25), fungal (26), or bacterial (27) antigenic attacks.
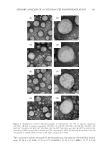
JOURNAL OF COSMETIC SCIENCE 320 Chronic UV exposure may induce skin cancer or suppress the immune system. Energy from UV is absorbed and is converted to biologically recognized signal. Photoreceptors that ab- sorb UV and initiate immunosuppression are epidermal DNA, trans-urocanic acid and membrane lipids (9). Mechanism of UV-induced immunosuppression has been depicted in Figure 6. Membrane lipid peroxidation and free radical formation. Membrane LPx and ROS are the me- diators of immunosuppression. Furthermore, activation of AP-1 (controls differentiation, proliferation, and apoptosis) and NF-κB (the protein complex that controls DNA tran- scription) leads to the formation of immune regulatory cytokines (28). ROS generated by UV exposure, leads to LPx and disturbance of redox potential. This initiates AP-1, NF-κB transcription and induces activation of cytokines (IL-4) (10). All these factors are respon- sible for systemic immunosuppression (9,29). Role of ROS in photoaging and immuno- suppression is explained in Figure 7. PHOTOCARCINOGENESIS Exposure of skin to UV radiation leads to a chain of bioeffects that contribute to photo- carcinogenesis. Mechanism of infl ammation and immunosuppression has been explained Figure 3. Histological appearance of photoaged and intrinsically aged skin. (A) Photoaged skin from the sunlight- exposed face, Elastica van Gieson staining (B) Photoaged skin from the sunlight-exposed face, hematoxylin–eosin staining (C) Intrinsically aged skin from the inner site of the upper branch of the same patient, Elastica van Gieson staining reveals a little reduction in elastic fi bers and (D) Intrinsically aged skin from the inner site of the upper branch of the same patient, hematoxylin–eosin staining. Adapted from Wlaschek et al. (7).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)