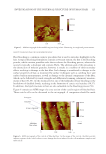
SOLVENT EFFECTS ON SPF AND BROAD-SPECTRUM PROTECTION 157 the solvent to that of the UV fi lter(s) has been shown to reduce UV fi lter degradation (26). The in vitro SPF of the homosalate–solvent mixtures ranged from 2.4 to 397.2 (Table II). The four highest SPFs were provided by diethylhexyl 2,6-naphthalate, butyloctyl salicy- late, ethylhexyl methoxycrylene, and polyester-8. The same four solvents resulted in the highest SPF for ethylhexyl salicylate as well in this case, the SPF ranged from 2.8 to 398.6. The butyl methoxydibenzoylmethane–solvent mixtures had higher SPF values than the UVB fi lter–solvent mixtures, except for ethylhexyl methoxycrylene. The in vitro SPF values ranged from 10.9 to 466.2 in this case. The in vitro SPF of the UV fi lter blend– solvent mixtures was higher in every case than the SPF of the individual UVB fi lter–solvent mixtures but lower than the butyl methoxydibenzoylmethane–solvent mixtures in many cases. The SPF ranged from 14.4 to 200.1. In the case of the UV fi lter blend–solvent mixtures, ethylhexyl methoxycrylene, polyester-8, diethylhexyl 2,6-naphthalate, and tris(PPG-3 benzyl ether) citrate resulted in the highest SPF values. Based on all the aforementioned observations, it can be concluded that structural ele- ments, including ester bonds, conjugated structure, aromatic rings, and –CN groups are important, but are not the only characteristics that can infl uence the SPF and SPF-boosting capability of a solvent. The photostabilizing effect of the solvents and polarity have also been shown to infl uence the λmax and molar absorptivity (19) of UV fi lters. Most solvents transmitted almost all light in the UV region, which is in correlation with the in vitro SPF results. Exceptions were C12-15 alkyl benzoate, diethylhexyl 2,6-naph- thalate, butyloctyl salicylate, ethylhexyl methoxycrylene, and polyester-8, which ab- sorbed light in both the UVB and UVA regions (Figure 1A). Ethylhexyl methoxycrylene barely had any transmittance in the UV region. Most mixtures of the UVB fi lters homo- salate and ethylhexyl salicylate covered effi ciently UVB and some of UVA-II but trans- mitted practically 100% of the radiation in the UVA-I region (Figures 1B and C). Exceptions were the mixtures with diethylhexyl 2,6-naphthalate, ethylhexyl methoxy- crylene, and polyester-8, which absorbed light even in the UVA-I region. It was noted that isododecane and a blend of cyclotetrasiloxane and cyclopentasiloxane when com- bined with ethylhexyl salicylate transmitted about 60% in the UVA range until 360 nm. Butyl methoxydibenzoylmethane transmitted about 5–10% in the UVB region and less than 5% in the UVA region (Figure 1D). This was an unusual fi nding considering that the literature classifi es butyl methoxydibenzoylmethane as a UVA fi lter therefore, no UVB protection was expected from it (7,27,28). The potential of butyl methoxydibenzo- ylmethane to absorb light in the UVB region is briefl y mentioned in one source (7). The UV fi lter blend was very similar to butyl methoxydibenzoylmethane, it transmitted about 5–10% in the UVB region and less than 10% in the UVA region (Figure 1E). We also determined which mixtures would pass the critical wavelength test. The UVB fi lters were not expected to pass the test however, in the case of both homosalate and ethylhexyl salicylate, the mixtures with ethylhexyl methoxycrylene had a critical wave- length 370 nm (387 and 386 nm, respectively Table III). The inherent UV-absorbing capacity was responsible for this unexpected result. Ethylhexyl methoxycrylene had a broad-spectrum protection as it can be seen in Figure 1. In the case of butyl methoxydiben- zoylmethane, all mixtures had a critical wavelength 370 nm, as it was expected given that butyl methoxydibenzoylmethane is a UVA fi lter. For the UV fi lter blend–solvent mixture, all mixtures had a critical wavelength 370 nm.
JOURNAL OF COSMETIC SCIENCE 158 P otential shift in the λmax was also studied. Having a shift in λmax could affect UV fi lter’s effi cacy and ability to provide optimal protection to the skin against UV rays. An ingre- dient may be a good booster or photostabilizer, but if it negatively affects the effi cacy of a UV fi lter, it is not recommended to be used. The λmax for homosalate and ethylhexyl salicylate was at 311 nm, and for butyl methoxydibenzoylmethane at 357 nm (29). The UV fi lter blend was expected to have two peaks, one correlating with the UVB fi lters, and the other with the UVA fi lter. Three solvents, including butyloctyl salicylate, ethylhexyl methoxycrylene, and polyester-8 had an absorption peak at 311, 312, and 311 nm, re- spectively. This fi nding is in correlation with the aforementioned results, referring to the inherent UV-absorbing capacity of the solvents. Diethylhexyl 2,6-naphthalate had three main peaks: the highest at 297 nm, the second highest at 350 nm, and the third at 335 nm. Two s olvents shifted the λmax of homosalate signifi cantly (p 0.05) toward shorter wavelengths (hypsochromic shift), including shea butter ethyl esters and diethylhexyl Figure 1. Transmittance spectra. (A) Solvents alone. (B) Homosalate–solvent mixtures. (C) Ethylhexyl sa- licylate–solvent mixtures. (D) Butyl methoxydibenzoylmethane–solvent mixtures. (E) UV fi lter blend–sol- vent mixtures.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)