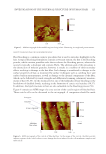
SOLVENT EFFECTS ON SPF AND BROAD-SPECTRUM PROTECTION 161 Overall, it c an be concluded that we did not observe a direct relationship between the chemical structure and IAG value of a solvent and the ability to cause a shift in the λmax. SPREADABILITY Sunscreens are often deemed greasy by consumers, which could lead to lower compliance (30). Emollients have been shown to contribute to the afterfeel of emulsions (21,31). We tested the spreadability of each solvent (most of which were emollients) to understand their potential effect on the skin feel of products. It should be noted that the goal of this project was not to create realistic sunscreens, we analyzed mixtures of solvents and UV fi lters to understand how solvents may change the in vitro SPF and broad-spectrum ab- sorbing properties of the UV fi lters. Formulators typically combine multiple solvents to achieve a desired product performance and skin feel. Analyzing spreadability can help Figure 2. Absorption spectra. (A) Solvents alone. (B) Homosalate–solvent mixtures. (C) Ethylhexyl salicylate– solvent mixtures. (D) Butyl methoxydibenzoylmethane–solvent mixtures. (E) UV fi lter blend–solvent mixtures.
JOURNAL OF COSMETIC SCIENCE 162 formulators make a decision about how much of a solvent should be used to boost the SPF, but at the same time, not to have a negative impact on the skin feel of the product. The positive p eak is a measure of fi rmness. Because we worked with liquid ingredients, the fi rmness values were considerably low in most instances. Firmness of skin cream gels is typi- cally in the range of 120–200 g (32), and skin creams could have even higher values (33). Two solvents had relatively higher numbers, still under 100 g, including mineral oil and Helianthus annuus (sunfl ower) seed oil (Table V). Only a few solvents had numbers above 200 g, including Ricinus communis (castor) oil, diethylhexyl 2,6-naphthalate, tris(PPG-3 benzyl ether) citrate, ethylhexyl methoxycrylene, and polyester-8, with the last two being above 3,000 g. The area under the positive curve is the measure of energy required to deform a sample to the defi ned distance. Firmness and positive area together indicate the spreadability of a sample. A higher fi rmness and more hardness work carried out indicate a less spreadable sample, whereas a lower positive peak and smaller area under the curve indicate a more spreadable sample. Firmness and overall spreadability values correlated well with each other. Ethylhexyl methoxycrylene and polyester-8 had by far the highest numbers, referring to their extremely poor spreading nature. The two most spreadable solvents were isododecane and ethanol. The negative peak indicates adhesive forces within the sample. The seven solvents that were considered fi rmer and less spreadable had the highest numbers for stickiness as well, with ethylhexyl methoxycrylene and polyester-8 being extremely sticky. The negative area under the curve gives an indication of adhesive- ness of a solvent. A sticky sample is typically described as a more cohesive. Cream gels typically have a negative peak in the range of 80–150 g (32), creams may have even higher values (33). Similar to the positive area under the curve, the negative area under the curve correlated well with the negative peak. The stickiest and most cohesive solvents were ethylhexyl methoxycrylene and polyester-8. Overall, many solvents that boosted the SPF had poor spreadability and a sticky nature, including diethylhexyl 2,6-naphthalate, ethylhexyl methoxycrylene, and polyester-8. Us- ing a higher amount of these solvents in a sunscreen could have a negative effect on the skin feel. In addition, ethylhexyl methoxycrylene and polyester-8 have a distinct color (yellow and amber, respectively), which could affect the color of the fi nished product when used in a larger amount. Butyloctyl salicylate on the other hand proved to be a rela- tively good SPF booster in this study without having a sticky and low spreading nature. CONCLUSIONS Tw enty-four so lvents commonly used in sunscreens and/or advertised by suppliers as sun- screen solvents, emollients for UV fi lters, photostabilizers, and/or SPF boosters were eval- uated for their ability to boost the in vitro SPF of two UVB fi lters and a UVA fi lter and a combination of these three fi lters. Critical wavelength, potential shift in λmax, and spread- ability were also analyzed. In this project , we used IAG to determine solubility. IAG indicated that most solvents would perform excellently with the UVB fi lters and UV fi lter blend, whereas they would only be good for butyl methoxydibenzoylmethane in most cases. Relying on IAG data only was not found to be a good approach in this study. Solvents, which achieved the highest in vitro SPF when mixed with UV fi lters (butyloctyl salicylate, diethylhexyl 2,6-naphthalate, ethylhexyl methoxycrylene, and polyester-8) shared multiple similar
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)