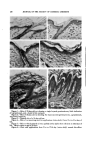
INFRARED C--H FREQUENCIES, THEIR SIGNIFICANCE 283 1943, 541. Farmer, E. H., and Sutton, D. A., Ibid., 19•4, 242. Bollond, J. L., and Koch, H. P., Ibid., 1945, 445. Atherton, D., and Hilditch, T. P., Ibid., 19•4, 105. (2) Patton, S., Kenney, M., and Kurtz, G. W., 7./lm. Oil Chemists' $oc., 28, 391 (1951). (3) Patton, S., and Kurtz, G. W., )'. Dairy Sci., 34, 669 (1951). (4) Bernheim, F., Bernheim, M. L. C., and Wilbur, K. M., )'. Biooe Chem., 174, 257 (1947). (5) Prill, E. A., Oil and Soap, 19, 107 (1942). (6) Myddleton, W. W., 7. Inst. Petroleum, 37, 45 (1951). (7) Rothenbach, E., 14•ochenschriftfiir Brauerei, 56, 273 (1939). THE INFRARED C-H FREQUENCIES AT 13.8•, AND THEIR SIGNIFICANCE* By F. C. NACHOD, E. T. HINKEL, JP.., and M. PRIZN^R Sterling-Winthrop Research Institute, Rensselaer, N.Y. As ^BSORPT•O•r B^m) in the 13.8/z region of hydrocarbons and its unusual behavior in a series of compounds have been the subject of theory and speculation for some time. A doublet at about 13.85/z was first pointed out by Thompson and Torkington (1) who ascribed it to a deformation vibration in the CH2 group of long straight-chain molecules. They also pointed out that liquid paraffins do not exhibit the doublet which appears in the spectrum of polyethylene. Molecular weight changes from 500 to 13,000 of the polymer did not alter the shape of the doublet significantly. Elliot, Ambrose, and Temple (2) found that using polarized infrared radiation caused the disappearance of the doublet and the formation of a single broad band. The disappearing component was ascribed to the rock- ing vibration mode of the CH2 groups across the chain. Simanouti (3) considered this perturbation (at 728•) as perpendicular to the chain axis. * Received for publication, Aug. 9, 1954. TABIra 1--CH• BA•ro API'EAR.-X•rCE Doublet Single Band Inflection Point (13.723, and 13.923,) (13.923,) (13.79u) Adurco Wax Palm Wax Bayberry Wax Barnsdall Amber Plio Wax Butyl Stearate Beeswax Polyethylene Glyceryl Monostearate Carnauba Wax Spermatine Wax Japan Wax Candelilla Spermaceti Lanolin Crown 1035 Stearic Acid Shellac I G Wax B unbleached StearylAlcohol I G Wax E Sugar Cane I G Wax OP Utah Wax I G Wax S Petrolatum I G Wax Z Vegetable Wax Ozokerite Raphia Wax White Ceresine Wax
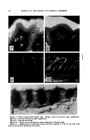
284 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 100 13 t• 14.5 Wavelength Figure 1.--Absorption spectrum of petrolatum: top curve, molten, 75ø bottom curve, solid 23 ø. 13 • 15 Wavelength Figure 2.--Absorption spectrum of I G Wax Z: ----- solid, room temp, ..... molten, 120 ø . Others have studied n-paraffins such as n-nonane, n-decane, and n-tetra- decane in crystalline state (4) and observed the doublet, and Sutherland (5) correlated it with the transition point of the substance. The doublet persists until the transition point is reached but is a singlet in the liquid state. Our own observations have borne out this observation. Figure 1 shows the 13-15• region of the infrared absorption spectrum of petrolatum, which essentially consists of n-hydrocarbons. Upon melting, the doublet dis- appears but can be recovered if the sample is placed shortly on a piece of
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)