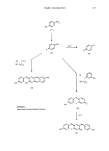
376 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS the maximum absorption wavelength of a substituted compound is calculated on the basis of the number and nature of substituents, each of which has a characteristic hypso- or bathochromic effect on the spectrum of the parent compound. This process has worked well with nitroanilines and nitrophenylenediamines (3). Molecular orbital calculations have been used to predict spectral maxima for a series of nitro- and dinitro-phenylenediamines (4) and several other classes of dyes (5). Generally good agreement is obtained for spectra measured in cyclohexane, except for compounds with severe steric crowding. Similar restrictions are found with the empirical rules, so the MO calculations, even though more soundly based, are of little better predictive value and neither method is useful in predicting absorption intensity. Presumably such studies will be extended and refined in the future. OXIDATION DYES Oxidation dye compositions have remained relatively unchanged over the last few years. However, the color forming reactions from oxidation dye mixtures have been extensively studied, and a generalized scheme is represented in Scheme 1. The chemistry of reactions with p-phenylenediamine (1: X = NH), has been known for some time (6). Recent work (7) has now extended these investigations to aminophenol (1: X = O). Kinetic studies have shown that p-aminophenol is oxidized to benzoquinone monoim- ine (2: X = O) which is slowly hydrolyzed to .p-benzoquinone (3) in alkaline solution. However, in the presence of other reactants, dye formation occurs. Thus, monoimine reacts with excess .p-aminophenol (8) to give the brown trinuclear dye (4: X = O) and with color couplers to give indo dyes, e.g., with phenols (9) to give blue indophenols (5: X = 7. = O Y -- H). In many cases, excessp-aminophenol will add to the 5-position of the indo dye, if vacant, to give tri-nuclear dyes such as (6). In general, these trinuclear dyes have significantly less distinct spectra than the all-nuclear indo dyes (5), and this may be a cause of the browns and yellows frequently obtained from p-aminophenol-based dyes. Bandrowski's base (4: X = NH) has been shown to have some mutagenic properties and its potential formation from dye solutions containing p-phenylenediamine had been considered a problem. However, kinetic studies show that the rate of self- coupling of.p-phenylenediamine (and of.p-aminophenol) is very much slower than their rate of coupling with typical color modifiers. Thus, in any commercial formulation, none of the self-coupled products can be formed. Application of the results of these in-vitro experiments to hair dye practice has not yet been fully developed. It is clear that dyes formed from individual couples in solution and on hair are similar, even though different oxidants may have been used to form them. This has been confirmed chromatographically (10) using dye formulations as well as synthetic mixtures of components. Of course, as the concentration of reactants is increased, the opportunity for bimolecular reactions such as (5) to (6) increases, and the number of products increases. In addition, commercial compositions consist of mixtures of dye ingredients blended to give the required shade, and the final color results from a complex combination of competing reactions. The author has used the published rate constants to predict relative product yields from mixtures of compo-
HAIR COLORANTS 377 (i) + (1) (ii) H202 HX '•NH2 (1) X •NH (2) HX (•) OH- (3) (i) Y '•Z H (ii) H202 Scheme 1: Reactions During Oxidation Dyeing. HX N (5) +(1) (6) XH
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)