412 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS A substantial improvement in the resolution of the proton magnetic resonance spectra can be obtained by the addition of so-called "shift reagents" (30-32). Typical shift reagents are complexes of the rare earth metals, europium and praseodymium with ligands such as 2,2,6,6-tetramethyl-3,5-heptanedione or 1,1,1,2,2,3,3-heptafluoro-7,7- dimethyl-4,6-octanedione. These are available commercially as standard reagents for NMR. They induce large changes in the chemical shifts of the NMR spectra of compounds which possess functional groups with free electron pairs capable of forming a coordinate bond with europium or praseodynium ions: for example, alcohols. The improved resolution results in the largest shift occurring for the protons closest to the coordination site. The first application of shift reagents to this field was the structural study of the ortho and para-alkyl isomers of phenol polyglycol ethers. The spectrum of the ortho isomers showed greater shift when complexed than the para isomers (33). Several researchers have reported on the use of HPLC for the characterization of surfactants (34,35). Studies have been done on esters of polyoxyethylene monododecyl ether prepared by reaction with 3,5-dinitrobenzoylchloride followed by HPLC of the ethoxylated species (36). Compounds containing more than 12EO units were difficult to chromatograph. Ethoxylated alkylphenols have also been separated using this esterification technique. The 3,5-dinitrobenzoylchloride was allowed to react for 30 minutes at 65øC in 20 ml of pyridine, followed by extraction in THF and separation by HPLC. A Lichrosorb RP-5, 5 um size column (Merck, Darmstadt, G.F.R.) with a mobile phase of acetonitrile/water (6:4) was used. The column was thermostatted at 50øC. Work has been reported on the separation of surfactant homologs by HPLC using an ODS/silica column (37). The authors were successful in obtaining separation of nine typical surfactants. Water/methanol adjusted to pH 2.2 with phosphoric acid and water/methanol containing 0.4M N•CI were used as the mobile phase. Further work on the separation of EO oligomers by HPLC has been done with isocratic and gradient elution (38,39). a-Olefinsulfonates (AOS) are one of the newest class of surfactant raw materials being used in cosmetic products. The surfactant is manufactured by sulfation of a-olefins obtained from either cracking of or polymerization of ethylene. The surfactants are thus mixtures of isomers and homologs having a wide distribution of chain lengths (C•0 to C20). Desulfonation of AOS has not been successful. However, successful GC analyses have been obtained after hydrogenation followed by the formation of the volatile sulfonyl chloride. The hydrogenation was followed by IR using the disappear- ance of the 965 cm -• band, and the GC analysis of the final mixture of the sulfonyl chlorides was performed using a 3% SE-30 column (40,41). The term "cationic surfactant" refers to compounds containing at least one hydro- phobic long chain alkyl group and a positively charged nitrogen. Generally referred to as "Quats," these materials are incorporated into cosmetic hair formulations imparting manageability and anti-static properties. Because of their inherent bacteriostatic properties, these compounds are also used as sanitizing agents, antiseptic agents, germicides, and fungicides. Temperature-programmed gas chromatography has been the general technique of choice for qualitative identification. The technique is hard to apply to quaternary ammonium salts without first fragmenting the compound into more volatile species. Attempts have been made to degrade certain quaternary ammonium compounds
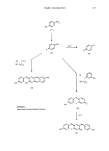
ANALYTICAL CHEMISTRY OF COSMETICS 413 directly on a hot alkaline column (42). Near quantitative (about 90%) degradation of alkyldimethylbenzylammonium halides into alkyldimethylamines and benzyl halides has been accomplished (43). The majority of quantitative methods available for this class rely upon the extraction of a relatively non-polar salt or complex formed by the surface active cation with an anion having a characteristic absorption in the visible or ultraviolet region (3). Many of these anions are the basic forms of acid-base indicators, and this principle is behind most of the methods used in the cosmetic industry. Some reports appear in the literature on the use of mass spectroscopy for the determination of long chain quaternary amines, offering greater information on the chemical nature of this class of surfactants (44,45). PRESERVATIVES An important aspect of cosmetic formulation is the incorporation and analysis of antimicrobial agents in raw materials and finished products. Early analytical work in this area used primarily thin layer chromatography for the separation and identification. Separation of twenty-five preservatives was accomplished by TLC on silica gel with a limit of detection of approximately 0.1-0.5 ug, using benzene-acetone as the solvent system (46). One report has looked at fifty antimicrobials divided into nine different groupings (47). It was found that silver nitrate could be used as a spray reagent for the identification of organic-halogen preservatives, and that gas chromatography could be successful in separating the silyl derivatives of phenolic compounds. Formaldehyde, an important preservative in cosmetic systems, is not detected by chromatographic methods but can be visualized easily by color reactions with a 1% solution of 4-amino-3-hydroquino-5-mercapto-l,2,4-triazole. This reaction can also be used for formaldehyde donors such as Bronopol ©, Dowicil 200 ©, Germall 115 ©, hexamine, and MDH Hydantoin ©. A method using fluorometric determination of formaldehyde- releasing cosmetic preservatives also appears in the literature (48). More recently, advanced instrumentation is proving useful for the separation and quantitation of these materials. A method has been published on a fast and rapid analysis for the methyl and propylparabens (49). It involves sample solubilization in the THF/EtOH mobile phase, and separation by HPLC. The detection levels for methylparaben are approximately 200 pg using UV detection at 254 nm. The same detection system using 45/55 acetonitrile/water mobile phase on a Sepralyte © C-18 column has been used to separate the methyl, ethyl, propyl, and butyl parabens simultaneously. Since these preservatives are often used in various combinations, HPLC affords a fast and rapid simultaneous analysis for these compounds. There is no doubt that instrumental techniques will continue to be explored for the analysis of preservative systems. FRAGRANCES Fragrance companies have traditionally led the industry in the development of methodology for the analysis and identification of compounds in essential oils. The greatest impact has come from the development of capillary gas chromatography. This
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)