416 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ogy which uses a thermal energy analyzer for the analysis of seven classes of cosmetic raw materials (68): ethanolamines, ethanolamides, monoethanolamine salts, trietha- nolamine salts, amphoteric compounds, quaternary ammonium compounds, and morpholine. NDEIA and other polar nitrosamines are very amenable to separation by reverse phase HPLC using either water or water/alcohol mobile phases (69). The water/alcohol mobile phase provides good solubility for the raw material, eliminating the need to perform multiple isolation steps prior to analysis. Quantitation of the nitrosamine is by UV detection. Methods are in the literature for the analysis of NDEIA in ethanol- amines, alkanoladines, and cosmetic products (70-74). Likewise, polography and conductivity detectors have been used for the analysis of NDoe1A (75,76). Since the initial findings of NDoe1A in cosmetics, other nitrosamines have also been shown to be present in trace quantities, and methodology has been developed for each nitrosamine (77). These have all been polar compounds, and both water soluble and oil soluble nitrosamines have been studied. Some methods are available for total nitrosamine in cosmetic raw materials and finished products however, these are generally not rapid and certainly not specific (78). By far the most absolute method for nitrosamine detection is mass spectrometry and, when possible, should be used to confirm the presence of the nitrosamine of interest. Researchers have reported the determination of NDEIA by first forming its disilyl derivative followed by GC/MS analysis (79). The second half of the nitrosamine problem, that of nitrite analysis, has also been explored. The standard colorimetric test developed by Greiss (80) and its subsequent modifications (70,81) can determine low ppb levels of nitrite, while derivative formation followed by fluorescent spectroscopy can measure in the picogram region (82). The CTFA has published nitrite methodology which is specific for cosmetic raw materials (83). More recently, trace analysis for 1,4-dioxane has become significant for the cosmetic analytical chemist. 1,4-dioxane can be present, at trace levels, in some types of ethylene oxide condensates, and this broad class of compounds is widely used in both the food and cosmetic industry. Presently, the "Birkel procedure" (84), which consists of vacuum distillation of a sample followed by gas chromatographic analysis, is the accepted validated procedure. The total analysis time per sample is 2-3 hours with a 0.5 ppm limit of detection. Several other methods have been generated through the CTFA (85). Samples of widely used cosmetic ingredients (i.e., sodium laureth sulfate, Polysorbate 60, and PEG-8) were chosen for study. A total of seven generally different analytical techniques were employed, including the Birkel and modified Birkel procedures. Other methods generated used GC/MS with perdeuterotoluene as an internal standard (86), purge and trap procedures followed by GC, direct OC injection, headspace GC, and atmospheric azeotropic distillation followed by GC (87,88). The CTFA study showed that these alternate procedures yielded results comparable to the Birkel method, except for the purge and trap technique. It was felt that with some additional methods development work, the purge and trap technique could also be improved to the point where it too would be satisfactory. The last couple of years has seen an increased concern within both industry and government in establishing the safety of the dyes and colors used in cosmetic products.
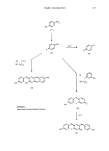
ANALYTICAL CHEMISTRY OF COSMETICS 417 This concern has recently been highlighted with respect to D & C Green (1,4-bis[(4-methylphenyl)amino]-9,10-anthracenedione), which is listed in the Code of Federal Regulations (CFR) for use in drugs and cosmetics. Because it is a certified dye, each commercially-prepared batch of this color is subject to FDA certification. This material is formed by reacting 1 mole of quinizarin with 2 moles of p-toluidine. D & C Green//16 has been shown to be safe for external use however, literature reports have demonstrated that p-toluidine is a carcinogen in mice (89). Residual amounts of reactants such as p-toluidine are commonly found in this color, and trace levels are unavoidably present even in highly purified reagent grade material. Until recently, there were no validated methods available for detecting trace levels of p-toluidine in D & C Green//16, thus the reluctance of the FDA to "permanently" list this color for use in consumer products. Several methods have since been reported (90,91) which can detect p-toluidine at the 500 ppb level and probably lower. The methods involve separation by HPLC followed by UV or fluorescence detection. This approach by the regulatory agencies of requiring analytical methodology for hazardous trace components in compounds shown to be safe is becoming standard, and the cosmetic analytical chemist will become more involved in developing trace analytical techniques for quality control of these raw materials. REFERENCES (1) L. R. Snyder and J.J. Kirkland, Introduction to Modern Liquid Chromatography, 2nd Edition (John Wiley & Sons, Inc., New York, 1979). (2) R. Namba, H. Nishiya, A. Shibamoto, Y. Morikawa, S. Tahara, and T. Mitsui, "Development of new automated analysis system of cosmetics by means of computer," IFSSC 12th International Congress, Paris, September 13-17, 1982. (3) Anionic Surfactants--Chemical Analysis, Surfactant Science Series, Vol. 8, J. Cross, Ed. (Marcel Dekker, New York, 1977). (4) J. Cross, Cationic Surfactants, E.Jungermann, Ed. (Marcel Dekker, New York, 1970), pp 419-482. (5) J. H. Jones, General colorimetric method for the determination of small quantities of sulfonated or sulfated surface active compounds,J. Assoc. O•c. Ag. Chemists, 28, 389-409 (1945). (6) G. P. Edwards, W. E. Ewers, and W. W. Mansfield, Determination of sodium cetyl sulfate and its solution in water, Analyst, 77, 205-207 (1952). (7) K. Burger, Methods for quantitative micro-determination and trace detection of surface active compounds. I. Detection and determination of very small amounts of anionics and cationics in aqueous solution, Z. Anal. Chem., 196, 15-21 (1963). (8) G. S. Buchanan and J. C. Griffith, Polarographic estimation of anionic detergents, J. Electroanal. Chem., 5, 204-207 (1963). (9) Laboratory Methods in Infrared Spectroscopy, 2nd Edition, R. G.J. Miller and B.C. Stace, Eds. (Heyden and Sons, Ltd., London, 1972). (10) D. Hummel, Identification and Analysis of Surface Active Agents by Infrared and Chemical Methods (Interscience Publishers, New York, 1962). (11) L.J. Bellamy, The Infrared Spectra of Complex Mokcuks, 2nd Edition (Methuen and Co., Ltd., London, 1958). (12) W. Brugel, An Introduction to Infrared Spectroscopy (Methuen and Co., Ltd., London, 1962). (13) N. B. Colthup, L. H. Daly, and S. E. Wiberley, Introduction to Infrared and Raman Spectroscopy (Academic Press, New York, 1964). (14) R. G. Sinclair, A. F. McKay, G. S. Myers, and R. N. Jones, The infrared absorption spectra of unsaturated fatty acids and esters,J. Am. Chem. Soc., 74, 2578-2585 (1952). (15) M. Aoki and Y. Iwayama, Determination of ionic surface active agents with dyes. IV. Applicability of fluorescein dyes and indicator, and stability of anionic detergents in solution, Yakugaku Zasshi (Tokyo), 80, 1749 (1960) (in Japanese). (16) W. E. Link, H. M. Hickman, and R. A. Morrissette, Gas-liquid chromatography of fatty derivatives. II.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)