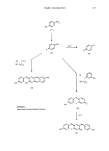
HAIR COLORANTS 377 (i) + (1) (ii) H202 HX '•NH2 (1) X •NH (2) HX (•) OH- (3) (i) Y '•Z H (ii) H202 Scheme 1: Reactions During Oxidation Dyeing. HX N (5) +(1) (6) XH
378 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS nents and, where possible, to compare these predictions with experimentally determined yields. So far results have been evaluated only for a single competition (i.e., one primary intermediate and two couplers, or two primary intermediates and one coupler). These results may not be directly translated into colors produced on hair from similar mixtures because factors such as diffusion rates of the various species also have to be considered. However, when dye yield in solution is compared with that predicted from the appropriate competing rate constants, generally good agreement is obtained. For example, when imine (2) was formed by ferricyanide [FC] oxidation in the presence of ten-fold molar excesses of two couplers, the ratio of the two indo dyes were, as predicted, for a first-order competition, in the ratio of the two coupling rate constants: coupler Iq dye [ 1] (2) • ß dye [2] coupler [2] then: dye [l]/dye [2] = k•/k2 This is demonstrated in Table I for competition of either p-phenylenediamine [PPD] or p-aminophenol [PAP] with m-phenylenediamine [MPD] and m-aminophenol [MAP]. Table I Competition for meta Couplers Under First-order Kinetic Conditions at pH 8.6 and 30 ø Relative Concentrations ' PPD PAP MPD MAP Expt % Para-MPD b Theory' 1 -- 10 -- 100 100 1 -- 0 10 0 0 1 -- 10 10 12 20 1 - 15 5 30 43 1 -- 17.5 2.5 49 63 -- 1 0 10 0 0 -- I 10 0 100 100 -- I 10 10 17 18 -- 1 15 5 31 40 -- 1 17.5 2.5 61 61 •Multiples of 9.3 x 10 -5 M. bDetermined spectrophotometrically. 'Rate constants: PPD-MPD, 298 PAP-MPD, 11 PPD-MAP, 1200 PAP-MAP, 49 1. mol-•s -•. Significant error is involved in determining experimental product yields spectropho- tometrically since the two products generally have similar spectra. Even so, the agreement between experiment and theory is good. A second example is shown in Table II, for competition of m-phenylenediamine and m-aminophenol for p-aminophenol (molar ratio 10:10:1) at various pH values.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)