HAIR COLORANTS 379 Table II Competition for PAP by MAP and MPD at Various pH Values and 30 ø % PAP - MPD Product pH Experimental Calculated 6.5 8O 68 7.1 65 62 7.8 34 3O 7.9 34 27 8.7 18 17 9.4 4 8 10.1 0 0 The trends predicted from the effects of pH on the individual coupling rate constants are reflected closely in the product yields from the competition experiments. Although these meta competition reactions are easy to evaluate, the corresponding para competitions are much more difficult because first-order kinetic conditions cannot be used. Thus a reaction stoichiometry such as a 10 PPD: 10 PAP: 1 coupler would show significant product formation from para self-coupling or cross-coupling or para addition to the coupled product. In addition, if a deficit of oxidant is used, some competition for oxidant will be evident and equilibria such as PAP q- Di-imine • Monoimine q- PPD .... (i) will be established, resulting in consecutive rather than competitive product formation (6). Product yields for the competitive system PPD - PAP - MAP are given in Table III. Table III Competition between PAP and PPD for MAP at pH 8.6 and 30 ø Relative Concentrations • % PAP Reacted % PPD Reacted % PPD Product PPD PAP MAP in Mixture Found Theory Found Theory 1 -- 1 100 0 0 100 100 1 0.5 1.5 74 50 50 75 75 1 1 2 52 48 50 53 50 0.5 1 1.5 43 57 50 86 100 -- 1 1 0 100 100 0 0 •Concentrations are multiples of 9.87 x 10 -• M 4 molar equivalents of FC added. Kinetic equations for competing second-order reactions have not been derived, but they are not needed for a quantitative understanding of these data. When a deficit of oxidant is used with a mixture of PPD and PAP, the PAP product is formed first even when the PAP coupling rate constant is very much less than the PPD rate constant. This is due to oxidation of PAP to monoimine by diimine by equilibrium (i), which effectively means that all the PAP is oxidized first. Any remaining ferricyanide is then used to oxidize PPD to diimine. Since diimine couples faster than monoimine under the experimental conditions, any remaining ferricyanide is used to
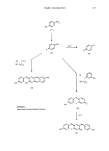
380 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS oxidize leucodiimine product to the final dye. Since there is no more ferricyanide, any further oxidations will use diimine initially (since it disappears the fastest) and then monoimine. Thus, in these experiments no more than 50% of the total PAP can be converted to monoimine dye. The theoretical yields of PAP and PPD dyes can therefore be calculated and are shown in Table III. The agreement between theory and experiment is excellent, thus supporting this interpretation of the mechanism of oxidation of mixtures of PPD and PAP. As a further extension of this theory, the product distribution should be independent of the coupler, since the product ratio depends only on the relative ratios of monoimine and diimine available for coupling and not on their coupling rates. This was confirmed experimentally with the couplers 1-naphthol, resorcinol, MPD and MAP where 53 + 8% PPD product was formed from a 1:1 PPD/PAP mixture, 74 + 7% from a 2:1 mixture, and 32 _+ 7% from a 1:2 mixture (theory: 50, 75, 33%). These results demonstrate the utility of the basic kinetic data in predicting results for more complex reactive mixtures. This procedure may be extended further, but better techniques must be used to analyze the mixtures experimentally. Polarography (11) has been used to investigate the oxidation-reduction properties of the primary intermediates used in oxidation dyeing this leads to an alternate way of determining coupling rate constants for these reactions. The second-order rate constant evaluated in this study for coupling p-phenylenediamine with m-aminophenol agrees very well with the value determined spectrophotometrically (12). During the review period there have been no significant introductions of novel primary intermediates although it has been suggested that a dyeing system can be based on tetra-aminopyrimidine (13) as well as several other N-heterocycles. In contrast, there has been intense activity in developing new couplers. Particular attention has been given to substitutes for 2,4-diaminoanisole, for which many analogues have been claimed. Probably the most useful of these is 2,4-diaminophenoxyethanol (14). Couplers that produce red shades have also been investigated and many m-aminophenols have been claimed in the patent literature. It is interesting to note that there has been a marked increase in claims for less damage from oxidative products. This is usually achieved by the addition of cationics such as quaternary amines (15) or polymers (16) which mask the effects of damage rather than reduce it. For many years, oxidative colors faded or changed shade under the influence of sunlight or perspiration. This problem is not as severe with current products. A recent survey (17) has compared the performance of most currently used dye components in relation to perspiration. It shows that coupled products from p-aminophenol had the least stability, while 4-aminodiphenylamine gave dyes with the greatest stability. Although most of the information was already available, the inclusion of several recently introduced components is useful. Chemical processes occurring during these color fading reactions have been shown to involve hydrolytic fission of the dye molecule at low pH, and cyclization to azine dyes at higher pH. These reactions are particularly easy with p-aminophenol-derived dyes (18,19).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)