j. Soc. Cosmet. Chem., 31,281-287 (November 1980) Analysis of 1,4-dioxane in ethoxylated surfactants M. L. STAFFORD, K. F. GUIN, G. A. JOHNSON, L. A. SANDERS, and S. L. ROCKEY, Shell Development Company, IVesthollow Research Center, P.O. Box 1380, Houston TX 77001 Received May 5, 1980. Presented at the Poster Session of the Society of Cosmetic Chemists' Annual Scientific Meeting, December, 1979. Synopsis An improved DIRECT INJECTION GAS CHROMATOGRAPHIC method has been developed for detecting 1,4-dioxane, an alleged animal carcinogen, in ethoxylated surfactants. It is a modification of ASTM Method D-3606-77 for the analysis of benzene in gasolines. The method has a detection limit of 0.5 mg/kg for alcohol ethoxylates and is suitable for routine quality control of commercial products. The ethoxylate sample, dissolved in chlorobenzene, is injected directly into a gas chromatograph equipped with two columns connected in series. The sample passes first through a column packed with a non-polar stationary phase, which separates the compounds according to boiling point. After the dioxane has eluted, the flow is reversed, flushing out the heavier ethoxylates and glycols. Dioxane and lighter components then pass through, and are separated, on a column packed with a highly polar stationary phase. Analysis of several commercial alcohol ethoxylates manufactured using a widely practiced, base-catalyzed procedure showed no detectable 1,4-dioxane using this method. INTRODUCTION Recently, 1,4-dioxane, an alleged animal carcinogen (1-5), was reported in trace amounts in certain polyethylene glycol food additives. In addition, there is the possibility that 1,4-dioxane is produced in the manufacture of alcohol ethoxylated surfactants. There are several methods currently used for 1,4-dioxane analysis. Birkel (6) of FDA used a closed-system vacuum steam distillation to concentrate and remove the 1,4-dioxane from Polysorbates -60 and -80 followed by gas chromatographic analysis of the distillate. The FDA procedure is tedious and time consuming as one operator can perform only two or three analyses per day. In general, other methods such as nitrogen sparging and trapping, and rotary distillation have shown poor repeatability. A headspace technique (7) being used has a detection limit of only 100 mg/kg. Another technique (7) could detect 0.5 mg/kg by sparging the sample with nitrogen and collecting the volatiles in a trap filled with a porous polymer adsorbent however, it is time consuming and requires large quantities of the sample. Some of the above difficulties have been overcome by the development of a direct injection gas chromatographic method with a detection limit of 0.5 mg/kg and the simplicity required for routine quality control of commercial products. 281
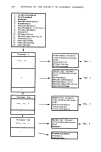
282 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS EXPERIMENTAL APPARATUS The gas chromatograph used in this work was a Varian Model 2440 equipped with a flame ionization detector. The schematic drawing in Figure 1 shows the column system and the flow configuration. The switching valve, a six-port medium tempera- ture valve with 1/8-in zero-volume fittings, was purchased from Valco Instruments A. Piping and Instrumentation A I J I •_ Vent ', , I ] Column A [ ] OVl01 & CW20M I I' X I B I 1 I Column B TCEP I [ ,Oven Boundary I I ,I Carrier Gas from Cylinder Flow Controller Pressure Indicator •O 6-Port Switching Valco Valve or Equivalent B. Flow Switching System Injection Port Forward Flow A Backflush A 3:1 10% OV101: 6% CW20M 1 6 3 25% TCEP •- Vent B 3:1 10% OV101: 6% CW20M Col• ._• Vent B 25% TCEP Figure 1. Column system set-up and flow configuration.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)