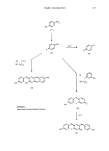
380 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS oxidize leucodiimine product to the final dye. Since there is no more ferricyanide, any further oxidations will use diimine initially (since it disappears the fastest) and then monoimine. Thus, in these experiments no more than 50% of the total PAP can be converted to monoimine dye. The theoretical yields of PAP and PPD dyes can therefore be calculated and are shown in Table III. The agreement between theory and experiment is excellent, thus supporting this interpretation of the mechanism of oxidation of mixtures of PPD and PAP. As a further extension of this theory, the product distribution should be independent of the coupler, since the product ratio depends only on the relative ratios of monoimine and diimine available for coupling and not on their coupling rates. This was confirmed experimentally with the couplers 1-naphthol, resorcinol, MPD and MAP where 53 + 8% PPD product was formed from a 1:1 PPD/PAP mixture, 74 + 7% from a 2:1 mixture, and 32 _+ 7% from a 1:2 mixture (theory: 50, 75, 33%). These results demonstrate the utility of the basic kinetic data in predicting results for more complex reactive mixtures. This procedure may be extended further, but better techniques must be used to analyze the mixtures experimentally. Polarography (11) has been used to investigate the oxidation-reduction properties of the primary intermediates used in oxidation dyeing this leads to an alternate way of determining coupling rate constants for these reactions. The second-order rate constant evaluated in this study for coupling p-phenylenediamine with m-aminophenol agrees very well with the value determined spectrophotometrically (12). During the review period there have been no significant introductions of novel primary intermediates although it has been suggested that a dyeing system can be based on tetra-aminopyrimidine (13) as well as several other N-heterocycles. In contrast, there has been intense activity in developing new couplers. Particular attention has been given to substitutes for 2,4-diaminoanisole, for which many analogues have been claimed. Probably the most useful of these is 2,4-diaminophenoxyethanol (14). Couplers that produce red shades have also been investigated and many m-aminophenols have been claimed in the patent literature. It is interesting to note that there has been a marked increase in claims for less damage from oxidative products. This is usually achieved by the addition of cationics such as quaternary amines (15) or polymers (16) which mask the effects of damage rather than reduce it. For many years, oxidative colors faded or changed shade under the influence of sunlight or perspiration. This problem is not as severe with current products. A recent survey (17) has compared the performance of most currently used dye components in relation to perspiration. It shows that coupled products from p-aminophenol had the least stability, while 4-aminodiphenylamine gave dyes with the greatest stability. Although most of the information was already available, the inclusion of several recently introduced components is useful. Chemical processes occurring during these color fading reactions have been shown to involve hydrolytic fission of the dye molecule at low pH, and cyclization to azine dyes at higher pH. These reactions are particularly easy with p-aminophenol-derived dyes (18,19).
HAIR COLORANTS 381 Despite the significance of these investigations, it has to be remembered that hair is not a passive substrate. While it allows the smaller dye precursors to diffuse in, it limits the diffusion of the larger dye molecules out. In addition, it provides a catalytic surface for peroxide decomposition, accelerating dye forming reactions but apparently protecting the dyes inside from the oxidative decolorization reactions they readily undergo in solution. Finally, hair can be dyed selectively in the presence of the scalp, although the larger pore sizes in the skin may allow for easier removal of dye during subsequent rinsing and shampooing. Thus, there are major areas of this dyeing process that are still incompletely understood. DIRECT DYES The chemistry of these dyeing systems is relatively simple since no chemical changes are involved during the dyeing process, and the dye color is transferred directly to the hair. Typical shades contain 10-12 different dyes, of which the most common are 2-nitro-p-phenylenediamines. A variety of N • and N 4 substituents has been added to the wide range of substituents already patented. Despite this, none seems to have a major advantage over the methyl or hydroxyethyl substituents previously claimed and widely used. Some substituted nitro-aminophenols have also been claimed (20). Of particular interest with this class of dye is their resistance to shampoo and rinsing. This property is carefully balanced in commercial shades and appears to be related to the dyes' structure and molecular weight (21). There has been a study of impurity content of these dyes in relation to product stability (22). Surprisingly, the nature of these impurities has not been determined, nor, in general, have purification techniques been widely applied in dye synthesis. It seems likely that purity of the dyes must affect many properties of the dyes, and more attention will be given to it in the near future. OTHER HAIR COLORING SYSTEMS Most hair coloring is performed with products of the previous two types. However, there is a significant market for products which perform more specialized functions and involve different chemistry. Development of a permanent hair coloring system based on direct dyes has been the subject of many patent claims over the years. An obvious advantage to the consumer is the direct transfer of solution color to the hair (unlike conventional permanents where the solution is initially almost colorless). A common approach is to use the reactive dyes developed for other fibers, e.g., wool (25), with curing conditions moderated for use on live heads. Dye is thus bonded covalently to the hair and cannot be removed during subsequent shampooing. The practicality of such systems remains to be proven since except for the relevant patents there is a distinct lack of published scientific information. Progressive dyeing systems are appropriate for the user who needs a gradual change in hair color. Most systems rely on aerial oxidation of the reactants. For example, a recent
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)