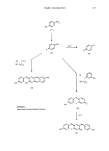
382 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS patent covers the auto-oxidations of 1,2,4-benzentriol with p-diaminobenzenes added to modify the shades (24). However, the metal salt-based compositions (e.g., lead acetate) are apparently still the only product for this type of dyeing effect, since no organic dye-based system is on the market. Patents have been issued at a steady rate for dyeing systems incorporating either colored resins (25) or natural melanin precursors (26). The lack of commercialization in these areas suggests major drawbacks both in performance and cost of such systems. In any case, the materials are not available in quantities where objective testing is possible. Significant advances in the technology are needed to overcome these problems. HAIR DYE TOXICOLOGY Over the last few years, this area has received substantial literature coverage which has recently been summarized by Corbett (2). During this time, most dye ingredients have been subjected to extensive toxicological evaluation including animal feeding studies. In general, adverse effects have not been observed and only three important dye ingredients were found to be carcinogenic in bioassay: 2,4-diaminoanisole, 2-nitro- p-phenylenediamine, and 2-nitro-4-aminophenol. Even if such studies are assumed to be valid, conservative risk assessment and human experience seem to support the position that there are no chronic untoward effects from the use of hair dyes. Thus, while such studies may reflect inherent properties of a dye ingredient, usage conditions must be considered. For example, the concentration of 2,4-diaminoanisole during the dyeing process has been monitored chromatographically (27). With a black shade, the concentration falls to around 10% of its original level within 15 minutes and to 5% within 30 minutes. This reflects rapid utilization of such intermediates and suggests that much less than the formulation amount is available during most of the dyeing step. In addition, dye must penetrate the scalp to cause many of the adverse effects. Skin penetration data on radiolabelled dyes after topical application show relatively low dye permeation rates which, however, depend on the area of application (28). Translating such results into actual dye usage is difficult. More recent work (29) has attempted to quantirate dye penetration under actual usage conditions. In essence, the data shows that with p-phenylenediamine, 2,4-diaminoani- sole, or HC Blue//1 less than 0.2% of the applied dye was absorbed by human subjects during the coloring process. The remainder was located in the hair (5-20%), or in the rinse water. One hopes that this type of study is extended to other common dye ingredients, so that a realistic picture of the toxicological risks associated with hair dye usage can be determined. CONCLUSIONS A theoretical understanding of the properties of dyes and the dyeing process has continued to develop as investigations of more complex and more realistic systems are undertaken. However, the interactions of such systems with hair and skin are still incompletely understood.
HAIR COLORANTS 383 REFERENCES (1) J. F. Corbett, Hair colouring, Rev. Prog. Coloration, 4, 3-7 (1973). (2) J. F. Corbett, Hair dye toxicity, in Hair Research (Springer-Verlag, Berlin, 1981), pp. 529-535. (3) J. F. Corbett, The electronic spectra of nitrobenzenes containing two electron donor substituents, Spectrochim. Acta, 23A, 2315-2332 (1967). (4) K-Y Chu andJ. Griffiths, Colour and constitution of nitro- and dinitro-p-phenylenediamines and their N-methyl derivatives,J. Chem. Soc. Perkin I, 1194-1198 (1978). (5) J. Griffiths, Practical aspects of color prediction of organic dye molecules, Dyes and Pigments, 3, 211-233 (1982). (6) J. F. Corbett, The role of meta difunctional benzene derivatives in oxidative hair dyeing. I. Reaction with p-diamines,J. Soc. Cosmet. Chem., 24, 103-134 (1973). (7) K. C. Brown and J. F. Corbett, The role of meta difunctional benzene derivatives and oxidative hair dyeing. II. Reaction with p~aminophenols,J. Soc. Cosmet. Chem., 30, 191-211 (1979). (8) K. C. Brown andJ. F. Corbett, Benzoquinone imines. Part 16,J. Chem. Soc. Perkin II, 308-311 (1979). (9) J. F. Corbett, Benzoquinone imines. Part VIII,J. Chem. Soc. B, 1502-1509 (1970). (10) M. J. Shah, Thin-layer chromatography of redox reaction products of oxidative hair dyes, J. Soc. Cosmet. Chem., 28, 259-271 (1977). (11) A. deLabbey, A. Baudry, and P. Bore, Voltammetry in oxidative hair dyeing, 12th IFSCC Congress, Paris, France, 13-17 Sept. 1982. (12) J. F. Corbett, Benzoquinone imines. Part IX,J. Chem. Soc. Perkin II, 539-548 (1972). (13) P. Berth, E. Lieske, D. Rose, and D. Schrader, The possibilities and limits of new hair dye systems, 11th IFSCC Congress, Venice, Italy, 22-25 Sept. 1980. (14) L'Oreal, U.S. Patent 4259261. (15) Clairol, U.S. Patent 4119399. (16)• L'Oreal, U.S. Patent 4047888. (17) I. Schwartz, J. Kravitz, and A. D'Angelo, Laboratory evaluation of some oxidation hair color intermediates, Cosmet. Tolietries, 94, (4), 47-50 (1979). (18) K. C. Brown andJ. F. Corbett, Benzoquinone imines. Part 15,J. Chem. Soc. Perkin II, 304-307 (1979). (19) K. C. Brown andJ. F. Corbett, Benzoquinone imines. Part 17,J. Chem. Soc. Perkin II, 886-889 (1981). (20) L'Oreal, U.S. Patent 4125601. (21) M. Y. M. Wong, Kinetics of dye rinse from bleached hair,J. Soc. Cosmet. Chem., 23, 165-170 (1972). (22) H. H. Tucker and I. Schwartz, Purification of some intermediates for permanent hair colors, J. $oc. Cosmet. Chem., 22, 139-151 (1971). (23) For a recent review see J. A. Maclaren and B. Milligan, IVool Science (Science Press, Marrickville, Australia, 1981), pp. 165-180. (24) Clairol, U.S. Patent 3920384. (25) e.g., Gillette, U.S. Patent 4182612. (26) e.g. Shiseido, U.S. Patent 3993436. (27) H. Gottschalck and P. Stachowiak, Quantitative tracing of 2,4-diaminoanisole in hair coloring products, Aertzl. Kosmetol., 10, 265-266 (1980). (28) F. N. Marzulli, D. M. Anjo, and H. I. Maibach, In-vivo skin penetration studies of 2,4-toluenediamine, 2,4-diaminoanisole, 2-nitro-p-phenylenediamine, p-dioxane and N-nitrosodiethanolamine in cosmet- ics, Food Cosmet. Toxicol., 19, 734-747 (1981). (29) H. I. Maibach and L.J. Wolfram, Percutaneous penetration of hair dyes, J. Soc. Cosmet. Chem., 32, 223-229 (1981).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)