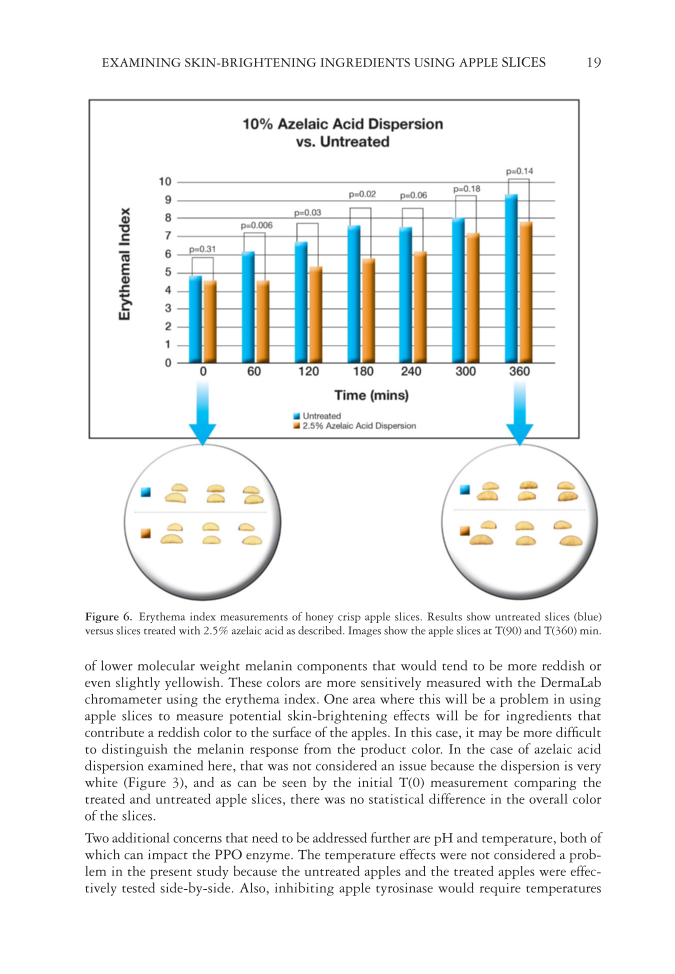
EXAMINING SKIN-BRIGHTENING INGREDIENTS USING APPLE SLICES 19 of lower molecular weight melanin components that would tend to be more reddish or even slightly yellowish. These colors are more sensitively measured with the DermaLab chromameter using the erythema index. One area where this will be a problem in using apple slices to measure potential skin-brightening effects will be for ingredients that contribute a reddish color to the surface of the apples. In this case, it may be more diffi cult to distinguish the melanin response from the product color. In the case of azelaic acid dispersion examined here, that was not considered an issue because the dispersion is very white (Figure 3), and as can be seen by the initial T(0) mea surement comparing the treated and untreated apple slices, there was no statistical difference in the overall color of the slices. Two ad ditional concerns that need to be addressed further are pH and temperature, both of which can impact the PPO enzyme. The temperature effects were not considered a prob- lem in the present study because the untreated apples and the treated apples were effec- tively tested side-by-side. Also, inhibiting apple tyrosinase would require temperatures F igure 6. E r ythema index measurements of honey crisp apple slices. Results show untreated slices (blue) versus slices treated with 2.5% azelaic acid as described. Images show the apple slices at T(90) and T(360) min.
JOURNAL OF COSMETIC SCIENCE 20 that would exceed 50°C, which was not the case. The pH effects are more of a concern as it is known, for example, that citric acid is a popular food treatment for sliced apples. How- ever, it has recently been reported that the effects of citric acid on PPO activity may not be solely related to pH as was noted in potatoes (17). Certainly, more studies are needed to make sure the infl uence of azelaic acid is not just a pH effect. However, azelaic acid has already been clinically proven to be an effective skin-brightening ingredient. Currently we are studying nonacidic skin-brightening ingredients to confi rm that the effects are related to the inhibition of PPO activity. CONCLUS ION We have reported here what we believe is the fi rst instanc e of the use of fruit slices to measure the effectiveness of a skin-brightening ingredient by direct topical application. Although it is easy to note that human skin and sliced apples are quite different and that, skin pigmentation is an extremely complex process, the model offers what may be a quick and easy way to screen numerous formulations or ingredient benefi ts for potential skin- brightening claims (18). The fact that human tyrosinase and fruit PPO are the same enzyme and would function similarly in human skin and fruits by controlling the rate- limiting step in the conversion of phenols to quinones in the production of melanin suggests that these models may work well for initial evaluation of ingredients that would impact early melanin production in the skin. It is likely no coincidence that ingredients popular in preserving food color, such as ascorbic acid and kojic acid, are also very popular skin- brightening ingredients. The methods described here are easy and could be run simply in any application laboratory that has access to a chromameter such as the DermaLab instru- ment used here. This preliminary summary describes in detail a potential for fruit slice measurements to be a way to examine skin-brightening technologies, while additional work is needed to defi ne the value and limitations of this technique. REFEREN CES (1) S. Parvez, M. Kang, H. S. Chung, C. Cho, M. C. Hong, M. K. Shin, and H. Bae, Survey and mechanism of skin depigmenting and lightening agents. Phytother Res., 20, 921–934 (2006). (2) B. B. Mishra and S. Gautam, Polyphenol oxidases: biochemical and molecular characterization, distri- bution, role and its control, Enzyme Eng., 5, 1–9 (2016). (3) M. Friedman, Prevention of adverse effects of food browning, Adv. Exp. Med. Biol., 289, 171–215 (1991). (4) G. B. Lum, B. J. Shelp, J. R. DeEll, and G. G. Bozzo, Oxidative metabolism is associated with physi- ological disorders in fruits stored under multiple environmental stresses, Plant Sci., 245, 143–152 (2016). (5) C. E. Deutch, Browning in apples: exploring the biochemical basis of an easily observable phenotype. Biochem. Mol. Biol. Educ., 46, 76–82 (2018). (6) M. D. Aytemir and G. Karakaya, “Kojic acid derivatives,” in Medicinal Chemistry and Drug Design, D. Ekinci. Ed. (InTech Europe, Rijeka, Croatia, 2012), pp. 1–27. (7) S. M. Son, K. D. Moon, and C. Y. Lee, Inhibitory effects of various antibrowning agents on apple slices, Food Chem., 73, 23–30 (2001). (8) Z. Zhao, G. Liu, Y. Meng, J. Tian, X. Chen, M. Shen, Y. Li, B. Li, C. Gao, S, Wu, C. Li, X. He, R. Jiang, M. Qian, X. Zheng. Synthesis and anti-tyrosinase mechanism of the substituted vanillyl analogues. cin- namate analogues. Bioorg Chem. 93, 103316 (2019). (9) A. Rouf, H. R. Naik, M. A. Beigh, V. Kanojia, S. A. Mir, S. Aafi a, Q. Ayaz, and U. Altaf, Enzymatic browning of apple and its control by chemical treatment: a review, Int. J. Food Sci. Nutr., 3, 81–88 (2018).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)