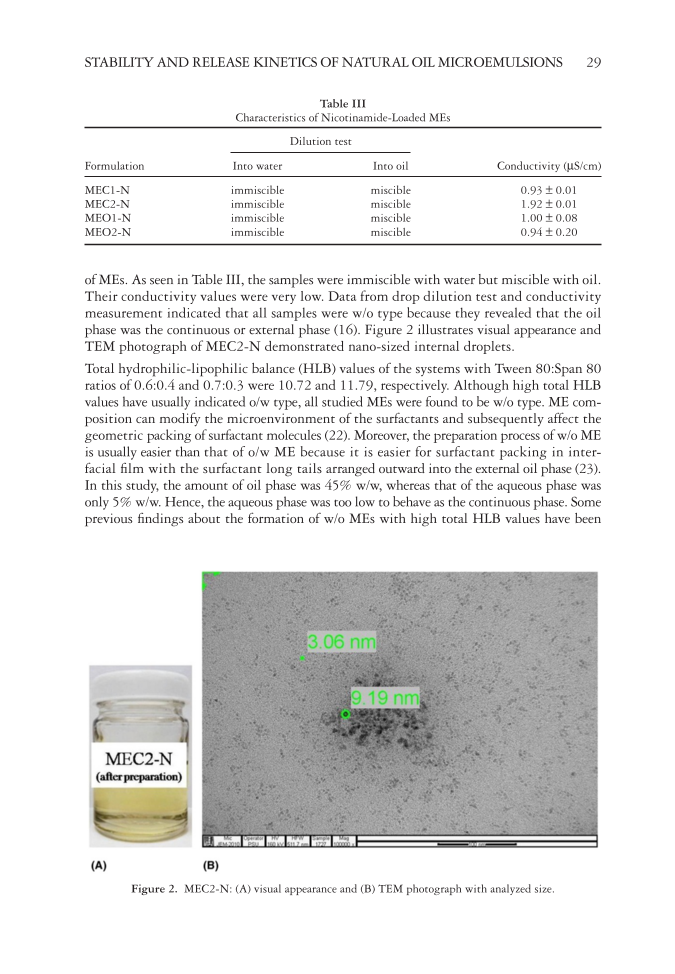
STABILITY AND RELEASE KINETICS OF NATURAL OIL MICROEMULSIONS 29 of MEs. As seen in Table III, the samples were immiscible with water but miscible with oil. Their conductivity values were very low. Data from drop dilution test and conductivity measurement indicated that all samples were w/o type because they revealed that the oil phase was the continuous or external phase (16). Figure 2 illustrates visual appearance and TEM photograph of MEC2-N demonstrated nano-sized internal droplets. Total hydrophilic-lipophilic bal ance (HLB) values of the systems with Tween 80:Span 80 ratios of 0.6:0.4 and 0.7:0.3 were 10.72 and 11.79, respectively. Although high total HLB values have usually indicated o/w type, all studied MEs were found to be w/o type. ME com- position can modify the microenvironment of the surfactants and subsequently affect the geometric packing of surfactant molecules (22). Moreover, the preparation process of w/o ME is usually easier than that of o/w ME because it is easier for surfactant packing in inter- facial fi lm with the surfactant long tails arranged outward into the external oil phase (23). In this study, the amount of oil phase was 45% w/w, whereas that of the aqueous phase was only 5% w/w. Hence, the aqueous phase was too low to behave as the continuous phase. Some previous fi ndings about the formation of w/o MEs with high total HLB values have been Table III Characteristics of Nicotinamide-Loaded MEs Formulation Dilution test Conductivity (μS/cm) Into water Into oil MEC1-N immiscible miscible 0.93 ± 0.01 MEC2-N immiscible miscible 1.92 ± 0.01 MEO1-N immiscible miscible 1.00 ± 0.08 MEO2-N immiscible miscible 0.94 ± 0.20 Figure 2. MEC2-N: (A) visual appearance and (B) TEM photograph with analyzed size.
JOURNAL OF COSMETIC SCIENCE 30 reported. For example, w/o MEs of palm kernel oil esters originated when the HLB values of surfactant mixtures were 10.7–13.9 because of large volume of hydrophobic portion according to the structural similarity between palm kernel oil esters and the surfactant used (21). ME systems composed of 45% w/w Tween 80 (HLB 15), 45% w/w Eutanol G (2-octyldo- decan-1-ol), and 10% w/w water were found to be of w/o type because of much lower frac- tion of the aqueous phase than that of oil phase (24). VALIDATION OF HPLC ANALYSIS No i nterfering peak was observed nearby the nicotinamide peak at the retention time of around 5.4–5.8 min as demonstrated in Figure 3. Other parameters, i.e., linearity of the calibration curve of nicotinamide concentrations between 1.25 and 40 μg/mL in terms of linear regression coeffi cient (r2), accuracy in term of %recovery, precision in term of %rel- ative standard deviation (%RSD), limit of detection (LOD), and limit of quantitation (LOQ) were summarized in Table IV. The results revealed that the HPLC technique was reliable according to the ICH guidelines (18). Hence, this analytical method was suitable for quantitative assay of nicotinamide. STABILITY OF NICOTINAMIDE-LOADED MES Phase separation was found in MEO 2-N after storage at all studied conditions for only 1 mo. The reason was unclear however, it may be caused by the effect of entropy changing of water molecules by attraction with a hydrophilic active ingredient on the instability of the interfa- cial fi lm (25). Darkened color was found in all nicotinamide-loaded MEs and their blank counterparts after being kept at 45°C for 3 mo. Discoloration of the samples was due to oxida- tion of either virgin coconut oil or olive oil in MEs. Similarities were found in previous reports of MEs and ME-based gels prepared from different oils and stored at high temperatures such as MEs prepared from Eutanol G at 45°C (10), MEs prepared from isopropyl palmitate at 45°C (11), and ME-based gels prepared from soybean oil stored at 60°C (17). Discoloration has adversely affected consumer confi dence therefore, nicotinamide-loaded MEs should not be stored at high temperatures. However, the color of virgin coconut oil and olive oil did not change after being stored under the same condition, i.e., at 45°C for 3 mo. Formation of w/o MEs could accelerate oxidation when compared with bulk oils because large surface area of MEs allowed interactions between water-soluble transition metals and oil (26). Viscosity values of non-separated nico tinamide-loaded MEs, i.e., MEC1-N, MEC2-N, and MEO1-N were measured every month during stability study as exhibited in Table V. All samples possessed rheological property as Newtonian fl ow (data not shown). The percentage of nicotinamide remaining in nonseparated MEs was summarized in Table VI. It could be observed that high storage temperature seemed to adversely affect chemical stability of nico- tinamide-loaded MEs. Stability of most vitamins, including nicotinamide, is infl uenced by many factors such as oxygen, light, and temperature (27). However, chemical instability of nico- tinamide-loaded MEs after being kept at 45°C for 3 mo was not obviously noted. Entrapment of a hydrophilic cosmetic ingredient in the internal aqueous droplets of w/o MEs has been found to enhance chemical stability because of the protection effect (28). Average nicotinamide remain- ing in the samples after storage at 4°C and RT for 3 mo exhibited tendency of chemical stability.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)