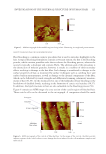
JOURNAL OF COSMETIC SCIENCE 128 appearance of the cuticle components. Again, swelling of the components in the cuticle cells due to solvent extraction could account for such an observation however, we should not discount the possibility that the effects of the grinding and polishing procedure used in this study could also lead to the granular appearance of the morphological components of hair as observed in Figures 2 and 6. However, this is unlikely as the granular structure is not observed in the cortex of hair. An AFM micrograph of the cortex of virgin and delipidated hair is provided in Figure 10. As compared with virgin hair, the image of delipidated hair clearly shows a delineation surrounding the macrofi brils. Possibly, such a result suggests that intermacrofi brillar material or lipids surrounding the macrofi brils are removed during the lipid extraction procedure. Similar to the features observed in Figure 1, the small pits seen as dark spots in the images, presumably correspond to sites where melanin granules were present, but then removed during the polishing step of the sample preparation procedure. Another interesting feature in the cortex of delipidated hair is the swollen nature of the macrofi - brils. Such a fi nding suggests that extraction of hair with solvents alters the structural morphology of the macrofi brils, although earlier work using the same extraction tech- nique as used in this report demonstrated that solvent extraction had no effect on the structural integrity of keratin intermediate fi laments as measured by differential scan- ning calorimetry (52). In TEM studies, the swollen nature of the macrofi brils in thiogly- colic acid–treated wool/hair was also found and attributed to the reduction of disulfi de bonds followed by reaction of osmium tetroxide with the sulfhydryl groups (1,62). The fact that this phenomenon also occurs in solvent-treated hair leads one to inquire about the source of the swelling behavior. CONCLUSIONS In this study, we demonstrate the use of cross-sectional analysis of human hair fi bers by AFM. The ultrafi ne structure of hair was delineated—confi rming previous work with Figure 10. AF M micrographs of the cortical region of (A) virgin and (B) delipidated hair.
INVESTIGATION OF THE INTERNAL STRUCTURE OF HUMAN HAIR 129 TEM—and new insights about the intermacrofi brillar region were introduced. Compari- sons were made between virgin and bleached hair as well as delipidated hair. In the hair cuticle, damage to the fi ber is especially noticeable in the endocuticle and is manifested in the form of low-density regions that likely correspond to disintegration of structural proteins. The cortical cells of bleached hair contain crevices, cracks, and cavities as a re- sult of the destructive bleaching procedure. Likewise, the intermacrofi brillar material is also degraded. In delipidated hair, the morphological components of hair (i.e., cuticle lamellar structures and macrofi brils) become swollen. In the past, such behavior was also observed in hair prepared for TEM studies and attributed to the reaction of osmium te- troxide (biological staining agent) with sulfhydryl groups of chemically reduced keratin fi bers. We are left to question the real source of swelling, which might be related to lipid structures in the fi ber or the effects of solvent extraction on protein structure. REFERENCES (1) G. Rogers, Electron microscopy of wool, J. Ultrastruct. Res., 2(3), 309–330 (1959). (2) J. Swift, The electron histochemistry of cystine—containing proteins in thin transverse sections of hu- man hair, J. Roy. Microsc. Soc., 88(4), 449–460 (1968). (3) M. Birbeck and E. Mercer, The electron microscopy of the human hair follicle. Part 1. Introduction and the hair cortex, J. Biophys. Biochem. Cytol., 3(2), 203–214 (1957). (4) M. Birbeck and E. Mercer, The electron microscopy of the human hair follicle. Part 2. The hair cuticle, J. Biophys. Biochem. Cytol., 3(2), 215–222 (1957). (5) M. Birbeck and E. Mercer, The electron microscopy of the human hair follicle. Part 3. The inner root sheath and trichohyaline, J. Biophys. Biochem. Cytol., 3(2), 223–230 (1957). (6) S. Gurden, V. Monteiro, E. Longo, and M. Ferreira, Quantitative analysis and classifi cation of AFM images of human hair, J. Microsc., 215(1), 13–23 (2004). (7) H. You and L. Yu, Atomic force microscopy as a tool for study of human hair, Scanning, 19(6), 431–437 (2006). (8) J. Smith, A quantitative method for analysing AFM images of the outer surfaces of human hair, J. Mi- crosc., 191(Pt 3), 223–228 (1998). (9) G. Poletti, F. Orsini, C. Lenardi, and E. Barborini, A comparative study between AFM and SEM imag- ing on human scalp hair, J. Microsc., 211(3), 249–255 (2003). (10) S. Breakspear, J. Smith, and G. Luengo, Effect of the covalently linked fatty acid 18-MEA on the nanotribology of hair’s outermost surface, J. Struct. Biol., 149(3), 235–242 (2005). (11) R. McMullen and S. Kelty, Investigation of human hair fi bers using lateral force microscopy, Scanning, 23(5), 337–345 (2001). (12) J. Smith, J. Tsibouklis, T. Nevell, and S. Breakspear, AFM friction and adhesion mapping of the sub- structures of human hair cuticles, Appl. Surf. Sci., 285(Pt B), 638–644 (2013). (13) C. LaTorre and B. Bhushan, Investigation of scale effects and directionality dependence on friction and adhesion of human hair using AFM and macroscale friction test apparatus, Ultramicroscopy, 106(8), 720–734 (2006). (14) T. Chen, H. Liu, and W. Yu, Investigation of nanotribological characterization of stretched European hair using atomic force microscopy, Adv. Mater. Res., 821–822, 274–277 (2013). (15) M. Sadaie, N. Nishikawa, S. Ohnishi, K. Tamada, K. Yase, and M. Hara, Studies of human hair by fric- tion force microscopy with the hair-model-probe, Colloids Surf. B Biointerfaces, 51(2), 120–129 (2006). (16) J. Smith and J. Swift, Maple syrup urine disease hair reveals the importance of 18-methyleicosanoic acid in cuticular delamination, Micron, 36(3), 261–266 (2005). (17) K. Jeong, K. Kim, G. Lee, S. Choi, T. Jeong, M. Shin, H. Park, W. Sim, and M. Lee, Investigation of aging effects in human hair using atomic force microscopy, Skin Res. Technol., 17(1), 63–68 (2011). (18) M. Richena and C. Rezende, Effect of photodamage on the outermost cuticle layer of human hair, J. Photochem. Photobiol. B Biol., 153, 296–304 (2015). (19) Y. Hessefort, B. Holland, and R. Cloud, True porosity measurement of hair: a new way to study hair damage mechanisms, J. Cosmet. Sci., 59(4), 303–315 (2008).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)