37
J. Cosmet. Sci., 75.1, 37–48 (January/February 2024)
*Address correspondence to Michael Pirrung, Michael.pirrung@ucr.edu
The Fallacy of Hyaluronic Acid Binding a Thousand Times its
Weight in Water
SCOTT BORCHERS AND MICHAEL C. PIRRUNG
Department of Chemistry, University of California, Riverside, California, USA (S.B., M.P.)
Department of Pharmaceutical Sciences, University of California, Irvine, California, USA (M.P.)
Accepted for publication January 01, 2024.
Synopsis
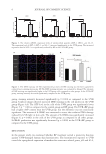
This study re-examined experimental reports and past literature of water binding by the humectant
hyaluronic acid, in comparison with another common humectant, glycerol, to critically evaluate the common
claim that hyaluronic acid binds a thousand times its weight in water, making it especially suited to be
a cosmetic moisturizer. Thermal gravimetric analysis (TGA) and differential scanning calorimetry (DSC)
were used to study aqueous solutions of glycerol and hyaluronic acid. A 0.1 weight %aqueous solution of
hyaluronic acid (the putative 1,000 times its weight) is a clear, flowing liquid, comparable to a 10 weight %
aqueous solution of glycerol. The melting point and melting heat of fusion of the hyaluronic acid solution were
indistinguishable from pure water, while both were reduced for the glycerol solution. This is as expected, as
colligative properties of aqueous solutions are proportional to concentration, and the polymer is at a much
lower molar concentration than glycerol. There is imperceptible freezing point depression by hyaluronic
acid, whereas that by glycerol is as expected. No experimental evidence was found for any special ability of
hyaluronic acid to bind water at the claimed level of a thousand times by weight. The origin of the fallacy that
it binds water at that level can be traced to older literature that has been misunderstood for the meaning of
binding, as compared to other physical properties such as hydrodynamics.
INTRODUCTION
Hyaluronic acid (HA) is a natural carbohydrate polymer that is a major component of the
human extracellular matrix. It is known for diverse physiological roles, including as the
lubricant in synovial fluid. In the form of a more widely available bacterial version, HA is
used as a humectant which enhances the water-holding capacity of the skin, in personal
care and cosmetic products, inter alia. Water is essential to normal skin function, and its
retention by the stratum corneum is facilitated by natural hygroscopic agents, including the
two compounds studied here, hyaluronic acid and glycerol.1 They enhance water absorption
from the dermis into the epidermis, and are also believed to aid absorption of ambient
water by the stratum corneum in humid conditions.2 Thus, the ability of a humectant to
bind water is intrinsic to its utility in cosmetic products, which gives this work its interest
to the cosmetic industry.
J. Cosmet. Sci., 75.1, 37–48 (January/February 2024)
*Address correspondence to Michael Pirrung, Michael.pirrung@ucr.edu
The Fallacy of Hyaluronic Acid Binding a Thousand Times its
Weight in Water
SCOTT BORCHERS AND MICHAEL C. PIRRUNG
Department of Chemistry, University of California, Riverside, California, USA (S.B., M.P.)
Department of Pharmaceutical Sciences, University of California, Irvine, California, USA (M.P.)
Accepted for publication January 01, 2024.
Synopsis
This study re-examined experimental reports and past literature of water binding by the humectant
hyaluronic acid, in comparison with another common humectant, glycerol, to critically evaluate the common
claim that hyaluronic acid binds a thousand times its weight in water, making it especially suited to be
a cosmetic moisturizer. Thermal gravimetric analysis (TGA) and differential scanning calorimetry (DSC)
were used to study aqueous solutions of glycerol and hyaluronic acid. A 0.1 weight %aqueous solution of
hyaluronic acid (the putative 1,000 times its weight) is a clear, flowing liquid, comparable to a 10 weight %
aqueous solution of glycerol. The melting point and melting heat of fusion of the hyaluronic acid solution were
indistinguishable from pure water, while both were reduced for the glycerol solution. This is as expected, as
colligative properties of aqueous solutions are proportional to concentration, and the polymer is at a much
lower molar concentration than glycerol. There is imperceptible freezing point depression by hyaluronic
acid, whereas that by glycerol is as expected. No experimental evidence was found for any special ability of
hyaluronic acid to bind water at the claimed level of a thousand times by weight. The origin of the fallacy that
it binds water at that level can be traced to older literature that has been misunderstood for the meaning of
binding, as compared to other physical properties such as hydrodynamics.
INTRODUCTION
Hyaluronic acid (HA) is a natural carbohydrate polymer that is a major component of the
human extracellular matrix. It is known for diverse physiological roles, including as the
lubricant in synovial fluid. In the form of a more widely available bacterial version, HA is
used as a humectant which enhances the water-holding capacity of the skin, in personal
care and cosmetic products, inter alia. Water is essential to normal skin function, and its
retention by the stratum corneum is facilitated by natural hygroscopic agents, including the
two compounds studied here, hyaluronic acid and glycerol.1 They enhance water absorption
from the dermis into the epidermis, and are also believed to aid absorption of ambient
water by the stratum corneum in humid conditions.2 Thus, the ability of a humectant to
bind water is intrinsic to its utility in cosmetic products, which gives this work its interest
to the cosmetic industry.