41 HyaluronicALURONIC acid water binding
heated and dried under high vacuum to remove all traces of water of hydration. Glycerol
was ACS certified reagent grade. Solutions of these materials (w/w%) were prepared in water
with resistance 50 mΩ from an in-lab water purification system. DSC was performed
using a Netzsch® DSC 214 Polyma (Selb, Germany). TGA was performed using a Netzsch®
TG 209 F1 Libra. DSC is a thermal analytical method where the difference in the amount
of heat evolved or required to change the temperature of a sample is measured as a function
of temperature. TGA is a method in which the mass of a sample is measured as the
temperature is raised. These tools provide information on phase transitions of solutions.
DSC was performed under nitrogen with two different temperature programs. The first
began at 20°C, chilled the sample to −40°C, and then returned the sample to 20°C. The
second began at 20°C, chilled the sample to −40°C, and then heated the sample to 150°C.
All temperature gradients were 5 K/min. The sample volume was ca. 10 µL. TGA was
performed beginning at 20°C with heating of the sample to 150°C at 5 K/min. The sample
volume was ca. 30 µL. All runs were performed in triplicate.
RESULTS
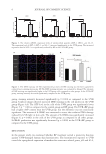
Several parameters (freezing heat of crystallization, melting heat of fusion, heat of vaporization,
and melting transition peak) were determined by DSC of HA and glycerol solutions and
pure water. The heat uptake/evolution as the sample is cooled from ambient to −40°C and
then warmed to a final target temperature is measured, from which the thermal parameters
mentioned can be derived. These data can enable various types of water (freezing free, non-
freezing bound, etc.) in a solution to be discerned. Results are summarized in Table I and
data are given in Figures 1–6. The freezing heat of crystallization should be the negative of
the melting heat of fusion, measuring the heat absorbed/produced as the solid-liquid phase
boundary is transited in each direction. That comparable absolute values are obtained for each
parameter for each solution shows there is no hysteresis in this phase change.
These values compare to literature values for water for the specific heat of fusion of 334 J
g−1 and for the heat of vaporization of 2,230 J g−1. The experimental values for water differ
from literature by 2–4%, suggesting this is the error in these measurements. The reduction
of melting heat of fusion and heat of vaporization in the glycerol solution are far greater
than the HA solution, which is as expected owing to the higher molar concentration of
glycerol. Both solutions give data significantly different from water.
TGA data are collected in Figures 7–9. Profiles for the HA solution show a mass loss of
100% at a temperature of 96.3°C, and the water control shows a mass loss of 100% at the
temperature of 99.6°C. The mass loss profiles of these two solutions are indistinguishable.
The 10% glycerol solution shows a mass loss of 89.4% at 100.6°C, and the mass loss profile
is significantly different from pure water.
Table I
Parameters for Solutions of HA and Glycerol Determined by DSC
HA Glycerol Water control
Freezing heat of crystallization (J g−1) −251 −222 −294
Melting heat of fusion (J g−1) 262 223 320
Heat of vaporization (J g−1) 2295 2036 2273
Peak of melting transition (°C) 1.2 −1.6 1.3
heated and dried under high vacuum to remove all traces of water of hydration. Glycerol
was ACS certified reagent grade. Solutions of these materials (w/w%) were prepared in water
with resistance 50 mΩ from an in-lab water purification system. DSC was performed
using a Netzsch® DSC 214 Polyma (Selb, Germany). TGA was performed using a Netzsch®
TG 209 F1 Libra. DSC is a thermal analytical method where the difference in the amount
of heat evolved or required to change the temperature of a sample is measured as a function
of temperature. TGA is a method in which the mass of a sample is measured as the
temperature is raised. These tools provide information on phase transitions of solutions.
DSC was performed under nitrogen with two different temperature programs. The first
began at 20°C, chilled the sample to −40°C, and then returned the sample to 20°C. The
second began at 20°C, chilled the sample to −40°C, and then heated the sample to 150°C.
All temperature gradients were 5 K/min. The sample volume was ca. 10 µL. TGA was
performed beginning at 20°C with heating of the sample to 150°C at 5 K/min. The sample
volume was ca. 30 µL. All runs were performed in triplicate.
RESULTS
Several parameters (freezing heat of crystallization, melting heat of fusion, heat of vaporization,
and melting transition peak) were determined by DSC of HA and glycerol solutions and
pure water. The heat uptake/evolution as the sample is cooled from ambient to −40°C and
then warmed to a final target temperature is measured, from which the thermal parameters
mentioned can be derived. These data can enable various types of water (freezing free, non-
freezing bound, etc.) in a solution to be discerned. Results are summarized in Table I and
data are given in Figures 1–6. The freezing heat of crystallization should be the negative of
the melting heat of fusion, measuring the heat absorbed/produced as the solid-liquid phase
boundary is transited in each direction. That comparable absolute values are obtained for each
parameter for each solution shows there is no hysteresis in this phase change.
These values compare to literature values for water for the specific heat of fusion of 334 J
g−1 and for the heat of vaporization of 2,230 J g−1. The experimental values for water differ
from literature by 2–4%, suggesting this is the error in these measurements. The reduction
of melting heat of fusion and heat of vaporization in the glycerol solution are far greater
than the HA solution, which is as expected owing to the higher molar concentration of
glycerol. Both solutions give data significantly different from water.
TGA data are collected in Figures 7–9. Profiles for the HA solution show a mass loss of
100% at a temperature of 96.3°C, and the water control shows a mass loss of 100% at the
temperature of 99.6°C. The mass loss profiles of these two solutions are indistinguishable.
The 10% glycerol solution shows a mass loss of 89.4% at 100.6°C, and the mass loss profile
is significantly different from pure water.
Table I
Parameters for Solutions of HA and Glycerol Determined by DSC
HA Glycerol Water control
Freezing heat of crystallization (J g−1) −251 −222 −294
Melting heat of fusion (J g−1) 262 223 320
Heat of vaporization (J g−1) 2295 2036 2273
Peak of melting transition (°C) 1.2 −1.6 1.3