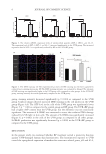
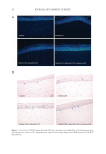
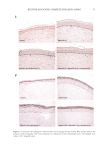
39 HyaluronicALURONIC acid water binding
Studies of high concentration HA solutions using DSC reached firm and consistent
conclusions about the amount of water strongly bound by the polysaccharide (i.e., non-
freezing water). Joshi found that up to 21 molecules of water were strongly bound to each
hyaluronic acid disaccharide monomer (i.e., repeat unit), and that the amount bound was
0.6 g H
2 O/g hyaluronic acid.13 Molecular dynamics simulations agree with the number of
water molecules per disaccharide monomer suggested by Joshi.14 Ikada found 0.51 g non-
freezing H
2 O/g hyaluronic acid, which corresponds to 11.5 molecules of water per repeating
HA disaccharide.15 He also made one of the few measurements of freezing bound water,
which he found to be 0.59 g H
2 O/g hyaluronic acid. Yoshida determined a value of 0.5 g
H
2 O/g hyaluronic acid for strongly bound water, which corresponds to 11 molecules per
repeat unit, and also measured the freezing bound water, which he found to be 0.5-1.8 g
H
2 O/g hyaluronic acid.16 Kučerík determined there are 17.2–19.1 water molecules bound
per polymer repeat unit, and that the amount bound was 0.77–0.86 g H
2 O/g hyaluronic
acid.17,18 One interesting feature of this earlier report is that water binding was studied as
a function of the HA molecular weight, and it was found to be approximately the same at
most molecular weights.
Multiple DSC studies of HA hydration used high concentrations which were needed to see
thermal transitions associated with bound water. A single study by Cowman used solutions
of HA as low as 0.5% concentration,19 approaching closest to the situation studied herein.
Cowman found an anomalously large amount of water associated with HA, calculated from
conventional analysis of the thermal analytical data. She explained this finding by the idea
that freezing bound water and non-freezing bound water are coincident at low concentrations.
Assuming based on past work 0.6 g H
2 O/g HA for non-freezing bound water, the data led
her to conclude contributions for freezing bound water are 44 g H
2 O/g HA.
Another, early method used to interrogate the amount of water bound by HA used ultrasound
to measure the speed of sound in HA solutions to determine their compressibility. Davies
found that in aqueous solution at 25°C, HA is “closely associated with not less than 9
molecules of water of hydration.”20 The mass of that number of water molecules would
be 144 g/mol, which compares to the 403 g/mol molecular weight of the hyaluronic acid
disaccharide. That corresponds to 0.36 g H
2 O/g hyaluronic acid. Jouon found that the
“amount of non-freezable water associated with the polysaccharide was about 0.7 g H
2 O/g
dry solids which corresponded to 15 water molecules per disaccharide unit.”21
While the foregoing reports used different methods to measure the amount of water
strongly bound to hyaluronic acid, which produced somewhat different results, they are all
similarly in the range of 0.36–0.86 g H
2 O/g hyaluronic acid. These results are all obviously
quite far from the 1,000 g per g of HA in the commonly made claim.
OH
HO OH
O
O
HO
OH
NaO2C O
HO
O
NH
OH
O
molecular weight
=403 amu
molecular weight
=92 amu
Chart 1. Chemical structures of the hyaluronic acid repeating disaccharide salt and glycerol. Hydrogen
bonding to water is possible for each N and O atom. Solvation by water is possible for the Na ion.
Studies of high concentration HA solutions using DSC reached firm and consistent
conclusions about the amount of water strongly bound by the polysaccharide (i.e., non-
freezing water). Joshi found that up to 21 molecules of water were strongly bound to each
hyaluronic acid disaccharide monomer (i.e., repeat unit), and that the amount bound was
0.6 g H
2 O/g hyaluronic acid.13 Molecular dynamics simulations agree with the number of
water molecules per disaccharide monomer suggested by Joshi.14 Ikada found 0.51 g non-
freezing H
2 O/g hyaluronic acid, which corresponds to 11.5 molecules of water per repeating
HA disaccharide.15 He also made one of the few measurements of freezing bound water,
which he found to be 0.59 g H
2 O/g hyaluronic acid. Yoshida determined a value of 0.5 g
H
2 O/g hyaluronic acid for strongly bound water, which corresponds to 11 molecules per
repeat unit, and also measured the freezing bound water, which he found to be 0.5-1.8 g
H
2 O/g hyaluronic acid.16 Kučerík determined there are 17.2–19.1 water molecules bound
per polymer repeat unit, and that the amount bound was 0.77–0.86 g H
2 O/g hyaluronic
acid.17,18 One interesting feature of this earlier report is that water binding was studied as
a function of the HA molecular weight, and it was found to be approximately the same at
most molecular weights.
Multiple DSC studies of HA hydration used high concentrations which were needed to see
thermal transitions associated with bound water. A single study by Cowman used solutions
of HA as low as 0.5% concentration,19 approaching closest to the situation studied herein.
Cowman found an anomalously large amount of water associated with HA, calculated from
conventional analysis of the thermal analytical data. She explained this finding by the idea
that freezing bound water and non-freezing bound water are coincident at low concentrations.
Assuming based on past work 0.6 g H
2 O/g HA for non-freezing bound water, the data led
her to conclude contributions for freezing bound water are 44 g H
2 O/g HA.
Another, early method used to interrogate the amount of water bound by HA used ultrasound
to measure the speed of sound in HA solutions to determine their compressibility. Davies
found that in aqueous solution at 25°C, HA is “closely associated with not less than 9
molecules of water of hydration.”20 The mass of that number of water molecules would
be 144 g/mol, which compares to the 403 g/mol molecular weight of the hyaluronic acid
disaccharide. That corresponds to 0.36 g H
2 O/g hyaluronic acid. Jouon found that the
“amount of non-freezable water associated with the polysaccharide was about 0.7 g H
2 O/g
dry solids which corresponded to 15 water molecules per disaccharide unit.”21
While the foregoing reports used different methods to measure the amount of water
strongly bound to hyaluronic acid, which produced somewhat different results, they are all
similarly in the range of 0.36–0.86 g H
2 O/g hyaluronic acid. These results are all obviously
quite far from the 1,000 g per g of HA in the commonly made claim.
OH
HO OH
O
O
HO
OH
NaO2C O
HO
O
NH
OH
O
molecular weight
=403 amu
molecular weight
=92 amu
Chart 1. Chemical structures of the hyaluronic acid repeating disaccharide salt and glycerol. Hydrogen
bonding to water is possible for each N and O atom. Solvation by water is possible for the Na ion.