THE PHYSICAL BEHAVIOR OF WATER-SOLUBLE CELLULOSE POLYMERS Bv J. B. BATDORF and P.S. }?RANelS* Presented December 4, 1962, New York City ABSTRACT The commercially available ionic and nonionic water-soluble cellulose polymers are sodium carboxymethylcellulose, hydroxyethylcellulose and methylcellulose. These polymers impart varying degrees of non-Newtonian behavior to solutions. The physical properties of aqueous and mixed aqueous solutions of these cellulose ethers are governed by the chemical nature of their solubilizing substituents and are dis- cussed with special emphasis on molecular structure. From a modest start in the early 1920's, water-soluble cellulose ethers now make an important contribution to modern industry. Today, the commercial product line is formidable, and new products are constantly being added. The manufacturing process for each of these derivatives usually involves: 1. Formation of alkali cellulose 2. Reaction with suitable organic reagent :3. Neutralization 4. Purification 5. Drying Typical reactions and products are shown in Fig. 1. Small amounts of water-soluble cellulose ethers dispersed in water greatly modify its over-all properties. The most obvious immediate change is an increase in the viscosity of the water. An interesting aspect of this vis- cosity increase is the fact that a single solution will appear to have a wide range of viscosities when different conditions of physical force are imposed on the solution. These conditions of physical force may be conveniently referred to as high, intermediate or low stress. For example, rolling or spreading a liquid as if it were an ointment or lotion would be high stress. On the other hand, after the liquid has been applied, gravity and surface tension control flow. These forces are conditions of low stress. Intermediate stress can be typified by pouring a liquid out of a bottle. Thus, if a solution of a water- * HerCules Powder Co., Wilmington 99, Del. 117
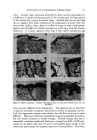
118 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS CELLULOSE + ALKALI + WATER ALKALI CELLULOSE + R ,, ALKYL R'-- ALKYL OR HYDROGEN X •- HALOGEN • ALKALI CELLULOSE I R-X O • ALKYL CELLULOSE ETHER R'-CH'-•H2 •--• HYDROXYALKYL CELLULOSE ETHER X-R'-COOH • CARBOXYALKYL CELLULOSE ETHER COMBINATIONS OF • (a) ALKYL HYDROXYALKYL CELLULOSE ETHERS ABOVE REAGENTS, (b) ALKYL CARBOXYALKYL CELLULOSE ETHERS (c) CARBOXYALKYL HYDROXYALKYL CELLULOSE ETHERS Figure 1.--Manuoeacture of cellulose ethers. soluble cellulose ether appears to be a viscous syrup as it is poured from a bottle, it will behave as a runny liquid when applied as a lotion yet, when high stress is removed it will instantly revert to a "molasses in January" consistency. These variations are pictured in Fig. 2. This type of flow behavior is referred to as pseudoplastic or non-Newtonian. There may also be a time-dependent consistency change usually called thixotropy. A dif- ferent molecular weight orviscosity grade of polymerwill behave in a similar fashion but to a different degree. The lower the molecular weight, the less change in viscosity will occur as stress conditions are varied. If viscosity does not change with stress, the solution is classified as NewtonJan. Low molecular weight cellulose ether solutions are less pseudoplastic or non- .. ß .• •?" : •. ::..:•: .: 2•-':. :.•: t50 Figure 2.--A single pseudoplastic fluid acts differently under different conditions of stress
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)