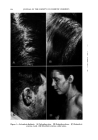
PHYSICAL BEHAVIOR OF WATER-SOLUBLE POLYMERS 121 CELLULOSE I O•H ,, H/O•H NONIONIC CELLULOSE CELLULOSE I I O•H O R t I I I I H•o/H H•o/H REQUIREMENT - POLYMER MUST BE ATTRACTED MORE TO THE SOLVENT THAN TO ITSELF. IONIC H CELLULOSE o / / o o / • I •H H I O•H I H\ I + o/" H/Oø"NJ a -- •H /o• H H Figure 5.--Forces for dissolving hydrocolloids in water. what the same manner as a jumping rope. In essence, the hydrophobic volume increases, causing the hydrophilic ether linkages to become less accessible to the water solvent. A similar mechanism is shown at the bottom of Fig. 7 for an alkyl cellulose ether. Since the hydroxyethyl- cellulose has terminal hydroxyl groups which are not affected by this increased hydrophobic sphere, it remains soluble even at the boiling point of water. In the alkyl cellulose ether, insufficient terminal hydroxyls are available to keep the polymer soluble as the hydrophobic volume of the alkyl substituent increases. At some point (or temperature) the derivative precipitates from water. If the water is made a poorer solvent, for in- stance by adding salt to it, we can demonstrate the same phenomenon with hydroxyethylcellulose as is exhibited with alkyl cellulose in plain water. Another interesting fact about the solubility of hydroxyethylcellulose is its relative solubility in solvents containing salts with multiple ionic charges. It has been found that hydroxyethylcellulose is less soluble in solutions containing multiple negative charges than in solutions containing AQUEOUS SOLUBILITY FACTORS POLYMER NO. OF HYDROPHOBIC NO. OF ETHER NO. OF -OH • / UNIT GROUPS CARB. ALKYL CELL. NONE 1.0
GROUPS 2.0
GROUPS/UN DECREASING HYD. ALKYL CELL. 1.5 .-.CH 2 -CH 2 - 1.5 3.0 THERMAL STABILITY HYD. ALKYL CELL. 2.5 '-'CH2-CH 2 2.5 3.0 IN WATER • ALKYL CELLULOSE • 2 -CH 3 • 2 • 1 Figure 6.--Aqueous solubility factors.
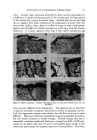
122 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Hi/O• H •/O• H i R,•O\ /O CH2• CH 2 H •o/H H •O / H .... \\ / / R• qCH 2 CH 2 /O\ CH 2 H H•/O•H /OJ•H 6 O•H / CH2• CH2 '"""•' •CH2_.,. CH 2 / (COLD-LOW ENERGY) .A (EXCITATION) H•o/H H I \ /! \ •.0• H • • •H • CH 2 /O• CH 2 CH 2 • • • • • .... J (HOT-HIGHER ENERGY, INCREASED HYDROPHOBIClTY). .--"I R-- O( CH3 Zt, •- R__ O•..CH3J '•XH/O•H ",• .,,,,J /o% Figure 7.--Factor governing interaction of ether links with water. O H H H •o/H /H • % / ,•H H J 'I'TI H o 'An o o--- C,a.t-- •H....-"""'•! \\ "" / H / '"'"' H I H / \ H/O•.H H H •o / Figure 8.--Suggested action of polyvalent ions. multiple positive charges. As shown in Fig. 8, it is proposed that the solvent molecules orient themselves so that fewer solvent hydrogens are available for associating with the cellulose molecules through its ether (C--O--C) linkages. These examples only suggest the versatility and sophistication of the water-soluble cellulose ethers. They serve to suggest that, whereas a single sample may not perform as desired in an end use, many other varia- tions of cellulose ethers exist which may turn the trick. The formulator is advised to seek information and assistance from the manufacturer of the cellulose ether in question before embarking on a program involving com- plex aqueous systems. (Received December 4, 1962)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)