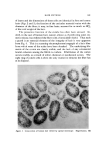
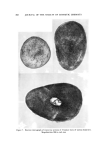
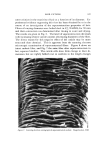
I. Soc. Cosmet. Chem., 22, 825-838 (December 9, 1971) Testing of New Compounds for Long-Term Toxicity ELIZABETH K. WEISBURGER, Ph.D. • Synopsis--Although the long-term testing of new compounds for CHRONIC TOXICITY may superficially appear to be a straightfmwvard matter, many factors can influence the outcome of such studies. These include the SPECIES and STRAIN of test animal, AGE at start of the tests, SEX, VEHICLE and ROUTE of administration, DIET, and other variables. Some materials which are used in cosmetic formulations have the capacity to inhibit or to enhance the response of animals to known carcinogens. Awareness of all these influences is necessary in designing meaningful long-term tests for toxicity. INTRODUCTION The National Cancer Institute (NCI) is faced with the responsi- bility of identifying carcinogenic hazards with the greatest possible ef- fectiveness, preferably prior to their widespread introduction into the human environment. Worldwide, thousands of new chemical com- pounds are proposed each year as entering the enviromnent (patent ap- plications) in actual practice, there may be hundreds introduced each year. The thorough testing of all these materials for safety would be- come an unmanageable task with respect to the space and budgetary re- quirements. Since the risk from each compound varies, depending on production, usage, chemical structure, extent of exposure, and character of the popu- lation, priorities for thorough testing of each material can be obtained through a process of successive screening. Eventually, by this scheme a * Experimental Pathology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Md. 20014. 825
826 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS yearly list of approximately 250 compounds, prime candidates for thor- ough study by NCI, will be produced. However, although this technique will systematize the selection of compounds for future testing, NCI has been engaged for sometime in a Bioassay Program, designed to determine whether certain materials may or may not be carcinogenic. In addition, under this program NCI scien- tists are attempting to develop bioassay methods which may indicate more quickly which compounds should be studied thoroughly. BIOASSAY M•TnOt)S Bioassay methods include cutaneous application (of most interest to the cosmetics field), oral administration, injection, implantation, and in- halation. These techniques have been reported in various monographs on testing of materials for safety (1-7). In these publications informa- tion regarding types ooe tests, numbers of animals to use, treatment, length of test, necropsy, and other factors are thoroughly discussed. Although cutaneous application appears to be a straigtforward method for the testing of cosmetic ingredients, there are many complicat- ing factors such as age, sex, species and strain of animal, route, vehicle, and diet which may alter the response of the test animals. Other factors being equal, the newborn or neonatal animal, especially the mouse, is generally more sensitive to most carcinogens (8, 9). Usually, but not al- ways, animals treated with carcinogens as newborns or infants show a higher incidence of some types of tumors in a shorter time in compari- son with adults (9) (Table I). Part of the effect may be ascribed to a lack of or lower levels of drug metabolizing enzymes in the newborn ani- mal. Consequently, the carcinogen is excreted less rapidly and has a greater opportunity to initiate the processes leading eventually to tumor Table I Starting Age and Effects of a Single Subcutaneous Injection of N-Nitrosodimethylamine in Rats • Tumor Incidence (%) Dose Age (mg/kg) Sex Renal Liver 1 day 21-25 M 40 44 F 40 44 12-15 weeks 25 F 8 0 Untreated M 0 0 F 4 0 • Data. from Della Porta and Terracini (9). The animals were kept until they reached 80 weeks of age.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)