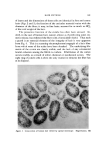
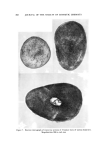
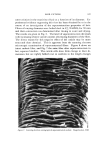
HAIR CUTICLE 843 ß o•. •.•,,• .•,.••,•:..: • ...... •'• •.•: ß . :• -• •.. '• • -&q{•,--.......•..•...••k : • ....... . .• ..... • •...•.• '• .•. '• ..• • •." ' '•5• •. •'.• ..... z... -- ß • .. ,• ß . •. • •-• • '•s . ..- .• . •.•.. ß . ,•... :.: • •:.• ß Figure 4. Scanning electron micrograph of hair abraded with a blade. Magnification, 1700 CHEMICAL COMPOSITION AND REACTIVITY Several attempts have been made to determine the chemical co•npo- sition of the cuticle cells after their separation by enzymatic or chemical methods (1-3). All these methods suffer because it is impossible to esti- mate the amount of degradation of the cuticle which had occurred during its separation from the cortex. In addition, almost all of the effort has been devoted to the analysis of wool with the result that little informa- tion is available regarding the cuticular material in hair. Bearing in mind the relative thickness of the cuticle layer in hair an abrasion technique seemed appropriate in this case. Small tresses of Cau- casian hair were scraped with a blade (single abrading stroke) and the scraped material was collected and carefully inspected under a micro- scope. In no case was any cortical contamination found, and it is very
844 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS likely that at the most only 3-4 cuticle cell layers were removed from the hair. The cuticle was hydrolyzed and its amino acid composition was de- termined. The amino acid content of the cortex was calculated based on the results of this analysis and the data for whole hair these results are shown in Table I. The analyzed hair samples were of medium coarse- ness, and for the purpose of this calculation it was assumed that the di- ameters of the individual fibers were uniform and within the range of 65-75 v. The amino acid composition of the cuticle clearly differs in many im- portant aspects from that of the cortex. The very extensive crosslinking of the cuticle by cystine and the high content of proline are indications of nonhelical organization of the protein chains. This implies isotrop- icity of structure in contrast to the strongly anisotropic fibrils present in the cortex. Some loss in the extent of electrostatic interactions (lower contents of basic and acidic amino acids) is compensated for by increased polarity due to the high contents of serine and glycine. Strangely enough, this intensification of polar interactions is accompanied by greater hydrophobicity. Table I Amino Acid Composition of Human Hair and Human Hair Cuticle Content in t•M/g Whole Cortex Amino Acid Hair Cuticle (Calcd) Cysteic acid 32 59 27 Aspartic acid 399 300 416 Threonine 554 412 580 Serine 967 1628 850 Glutamic acid 916 848 930 Proline 588 900 532 Glycine 437 836 368 Alanine 347 500 370 Half-cystine 1435 1880 1350 Valine 405 644 374 Methionine 13 39 9 Isoleucine 174 186 172 Leucine 457 404 466 Tyrosine 158 134 162 Phenylalanine 124 115 126 Lysine 196 331 172 Histidine 62 53 65 Arginine 466 289 496
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)