ASSIMILATION OF INGREDIENTS BY MICROORGANISMS 853 Table II List of Tested Materials Chemical Materials a Abbreviation Hydro- Liquid paraffin-SHP 160 L.P.-1 carbons Liquid paraffin-Brandol L.P.-2 Vaseline V Solid paraffin S.P. Multiwax M.W. Camell ian • Cam. Squalane S.Q. Silicones Silicone KF-96 c KF-96 Silicone KF-56 a KF-56 Alcohols Oleyl alcohol O1 A1 Stearyl alcohol St-A1 Hexadecyl alcohol HDA Propylene glycol P.G. Polyethylene glycol 400 PEG-400 Esters Isopropyl myristate IPM 2-Hexyldecyl myristate E-103 Diisopropyl adipate IPA Di(2-hexyldecyl) adipate E-201 Fatty acids Oleic acid O1-Ac Stearic acid St-Ac The commercial grade was used without further purification. Dimer dipentene manufactured by Japan Fine Chemicals, Koube, Japan. Dimethyl polysiloxane manufactured by Shinetsu Chemical Co. Ltd., Tokyo, Japan. Methyl phenyl polysiloxane manufactured by Shinetsu Chemical Co. Ltd., Tokyo, Japan. Lapisol* solution and pipetted into several 100-ml Erlenmeyer flasks containing g0 ml of culture medium. The flasks, set onto a reciprocal shaker, were incubated for 10 days at 30øC. When larger quantities were necessary for the study, a 500-ml flask containing 150 ml of medium was used. The fungi were treated in a somewhat different manner. Spores, which had been incubated at 25øG for 10 days and suspended in 0.006% Lapisol solution, were inoculated into 50-ml conical flasks containing 20 ml of media. The flasks were held stationary and incubated for 20 days at rotan temperature. Determination of Growth of Organisms In order to determine the growth of bacteria and yeasts, a cell suspen- sion was prepared as indicated in Fig. 1. The optical density at 660 m• * Anionic surfactant manufactured by Nippon Oil 8c Fats Co., Ltd., Tokyo, Japan.
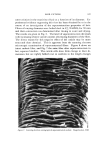
854 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Broth (2ml) f 0.006 Y• Lapisol Acidified Agitated & Centrifuged I I Supernatant Ppt Washed with Petr.ether Centrifuged I I Ether Ppt - Suspended in 5ml, H20 ICell Suspension] Figure 1. Preparative procedure of cell suspension Broth • Acidified Centrifuged I • Supernatant ppt --Extracted with CHCI• I Water layer Chloroform layer Dried with Na2S04 (overnight) Evaporated in vacuo Sample Figure 2. Extractive procedure of oil parts was measured by a spectrophotometer for each of the cell suspensions. This result indicated the relative concentration of organism present be- fore incubation. Following incubation, the broth was treated as shown in Fig. 2. The conversion of substrates due to the organism was studied by gas- liquid chromatography and infrared absorption. A Varian Aerograph Series 1200 gas chromatograph was used. It was fitted with a 3 ft X 1/s in. column using Chromosorb AW (80/100) as the solid support. The stationary phase consisted of SE-30 (3%) and nitrogen at 30 ml/min was used as the carrier gas. The unit was fitted with a FID detector and was temperature programmed at 100øC with a rise of 8øC per minute.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)