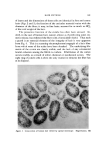
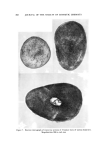
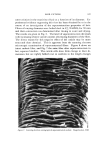
HAIR CUTICLE 849 Table III Resistance to Extension of Hair of Varied Diameter Yield Stress (g/den) Breaking Stress (g/den) Cuticle/ Diameter Cortex pH 7 pH 7 (u) Ratio s 65% RH buffer 65% RH buffer 50.2 0.41 0.93 0.42 2.07 1.24 65.1 0.30 0.92 0.37 1.88 1.02 84.8 0.22 0.93 0.37 1.85 1.17 Calculated assuming 4-• thick cuticular band. linked histological component amounting often to 25% of the fiber sub- stance could be without an effect. Indeed, our preliminary experiments on fibers of varying diameters tend to substantiate a view that the re- sponse of the cuticular material to mechanical stimuli (in extension mode at least) can well be as important as that of the cortex (Table III). The mechanical properties of dry fibers show little difference with the change ot} the cuticle/cortex ratio while on wetting the fine fibers ap- pear the strongest. This may be due to a lesser hydration of the cuticle as compared to cortex, although the effect of medullation in the coarse fibers cannot be neglected. Some additional work in this area with par- ticular attention being given to the torsional characteristic of hair appears to hold great promise. CONCLUSION In this brief discourse we have attempted to draw the attention of workers in the field of keratin chemistry and physics to some aspects of the structure/property relationship which tends often to be disre- garded or treated superfluously. Owing to a strategic position in the architecture ot} hair, the cuticle offers a rewarding subject for study with all its structural and chemical implications. We have barely touched upon some physico-chemical aspects of its function. Viewing the results in retrospect, it may well be that the known resistance of fine hair to many chemical treatments, but particularly to waving, can conceivably be accounted for by the cuticle factor alone. ACKNOWLEDGMENT We thank Mr. Don Coble for preparation and electron microscopic examination of hair specimens shown in Figs. 1 and 3. (Received February 17, 1971)
850 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS REFERENCES (1) Geiger, W. B., Scale substance of wool, J. Res. Nat. Bur. Stand., $2, 127 (1944). (2) Lindley, H., Chemical constitution of keratin, Nature, 160, 190 (1947). (3) Bradbury, J. H., Chapman, G. V., Hambly, A. N., and King, N. L. R., Separation of chem- ically unmodified histological components of keratin fibers and analyses of cuticles, Ibid., 210, 1333 (1966). (4) Bigelow, Charles C., The average hydrophobicity of proteins and the relation between it and protein structure, J. Theor. Biol., 16 (2), 187 (1970). (5) Tanford, Charles, Contribution of hydrophobic interactions to the stability of the globu- lar conformation of proteins, J. Arner. Chem. Soc., 84, 4240 (1962). (6) Goldsack, Douglas E., Relation of the hydrophobicity index to the thermal stability of homologous proteins, Biopolymers, 9, 244 (1970).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)