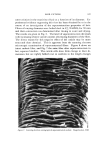
TERMINOLOGY OF EMULSION BEHAVIOR 819 Demulsification (coalesced drops constitute a clear disperse phase) Reversible _ Freshly prepared ._.. Flocculation Creaming . emulsion (separate..-. (two or more (droplets disperse droplets) /droplets form concentrate either /flocs) at the top or bottom and the droplets don't lose their identity) J ,Coalescence J increases the rate •/' of irreversible creaming Coalescence (flocs --,- Irreversible form single drops) creaming (such irreversible cream consists of coalesced particles and so the original disperse droplets have lost their identity. But such cream can be reversed to the coalesced emulsion.) Figure 1. Overall possible behavior of an emulsion system richer phase is lighter than the dispersion medium, the phenomenon is termed "creaming" if it sinks to the bottom, it is called "sedimentation." Some of the vital characteristics of the creaming phenomenon are: (a) in creamed emulsion, the particles do not lose their identity (b) creaming can be totally averted by adjusting the densities such that those of the dispersion medium and the disperse phase are equal (c) while creaming is not a strict revelation of emulsion instability, as regards the separation of clear disperse phase, still it has a strong interplay with other forms of instability. Creaming brings the disperse droplets in close proximity, thereby rendering the situation conducive to demulsifi- cation, through various stages as shown in Fig. 1 (d) flocculation enhances the rate of creaming, if there are appreciable density differences between the dispersion medium and the floccules. In creaming accelerated by flocculation the particles do not lose their identity and the creamed emulsion can be restored to its original form. In fact, this form of cream- ing can be termed "reversible creaming" (e) coalescence also increases the rate of creaming if there are appreciable density differences between the dispersion medium and the coalesced drops. Such creaming phe- nomenon differs from that accelerated by flocculation in the sense that the cream containing coalesced drops cannot be restored to the original form of emulsion. So it might well be designated "irreversible cream- ing." It is obvious from the above that "creaming" does not, in any case, represent the phenomenon of coagulation, as it does not conform with the requirements set forth for "coagulation" in the case of sols. These
820 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS are two widely different concepts and should not be confused with each other. Furthermore, workers interested in the quantitative and mech- anistic aspects of demulsification should resist the temptation to measure the rate of creaming. Flocculation This is the second and, unfortunately, the most disconcerting repre- sentation of reversible instability. When two or more droplets approach each other and form an aggregate (in which the droplets have not lost their identity), this poses a problem. How should this aggregate and the aggregation process be denominated? In the case of sols, this dif- ficulty is obviated by differentiating the aggregation process into two distinct processes-fiocculation and coagulation--depending upon certain requirements. In most ot• the aggregated emulsions, the droplets are separated by the intervening medium and the aggregated emulsions can be restored to their original state (17, 18). Thus, the process of aggregation of emul- sion droplets should be termed "fiocculation" not coagulation this is in accordance with the requirements prescribed earlier. The above discus- sion suggests that the term "coagulation" should not be used to describe emulsion behavior its use should be restricted to solid particle suspen- sions only. Furthermore, the statement made by Ecanow et al. (1) is not justified because fiocculated emulsions do exist. In the case of sols, two different mechanisms were proposed for the process of fiocculation however, in emulsions, fiocculation can take place by one of the followin• three mechanisms: (a) by reduction of the zeta potential and can be termed "electrostatic" or "ionic" fiocculation (b) by adsorption bridging and (c) by chemical bridging. The first category of flocculation is analogous to coagulation in sols only in the sense that both are brought about by the reduction of zeta potential. The mech- anisms (b) and (c) correspond to those proposed for the flocculation in sols, as discussed earlier. The process of flocculation in emulsions differs in some respects from that in sols, and the important characteristics of fiocculation in emulsions are the following: (a) As regards creaming, the riocs behave as single drops and the rate of "reversible creaming" is accelerated if the density of the riocs is appreciably different from that of the dispersion medium. In such creaming the particles have not lost their identity. (b) As the droplets are in close contact, fiocculation is precursor to the process
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)