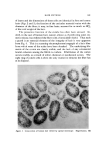
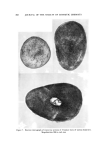
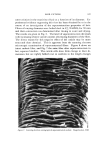
TERMINOLOGY OF EMULSION BEHAVIOR 817 Chemical Instability This form of instability refers to the chemical changes, in either the liquid phases or the emulsifying agents, which produce an intrinsic emulsion instability or the tendency towards demulsification or breaking of the emulsion. The two kinds of instabilities (chemical and physical) are attributed to widely different causes and the chemical instability may precede the physical instability. Still, the nomenclature recommended for the description of the disperse droplets will be the same. Physical Instab ility Generally, this form of instability relates to the shelf life of the emulsion when only the gravitational forces are considered. Although the exact correlation between the accelerated ultracentrifugal and the shelf life instability is not established (7-9), it is not unreasonable to include both forms of instability under the generic title "physical in- stability." After this introduction, the various terms used for representing emul- sion behavior are described below. The author is strongly inclined to propose that an emulsion can show instability--especially physical insta- bility-in one of the two ways: reversible instability or irreversible in- stability. The present classification is different and more comprehensive than those proposed earlier (2). Reversible instability can be further di- vided into: (a) creaming or sedimentation and (b) fiocculation. Irre- versible instability can be manifested by (a) coalescence, (b) demulsifica- tion or breaking, and (c) inversion. In the present text, demulsification and breaking are used as synonyms and interchangeably, as these repre- sent the same process, as shown later. The concept of "reversibility" in emulsion instability, it is hoped, will prove useful in comprehending better the mode of aggregation of disperse emulsion droplets. Before proceeding further, it is important and necessary to point out that some of the confusion in emulsion systems arises from the nomenclature used in solid particle suspensions. A short synopsis of the accepted termi- nology in sols is in order. TERMINOLOGY USED TO DESCRIBE THE BEHAVIOR OF SOLID PARTICLES IN SOLS La Met (10) pointed out that the terms "coagulation" and "floccula- tion" (both pertaining to the aggregation of sol particles) should be de- fined clearly and discriminately. According to La Met, the term coagu-
818 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS lation comes from the Latin "coagulare" meaning to drive together. This is an appropriate term for the effect of neutral salt on a sol, where the mechanism generally accepted today results from the reduction of the repulsive potential of the electrical double layers surrounding the particles. Flocculation comes from the Latin "fiocculare" meaning to form a floc i.e., a tuft of wool or highly fibrous structure. While the new ideas advocated by La Met and other groups (10-12) are still con- sidered controversial (13), the new vocabulary and the underlying ideas are being published (14). I am inclined to agree with the ideas of La Met and other groups. According to Ecanow et al. (1), in flocculation a network structure results. This, in turn, produces large bulky aggre- gates referred to as flocs or floccules. These fioccules contain varying amounts of entrapped medium. The network structure referred to can occur by means of adsorption bridging (15) and chemical bridging (11, 12, 16). The processes of flocculation and coagulation can be differentiated on the basis of multifold criteria (1, 10) but it is clear (1) that flocculation is reversible and the floccules contain entrapped medium, whereas coagu- lation is irreversible and the coagula are compact structures with no entrapped medium. It should be pointed out that I have regarded these criteria--reversibility, unchanged identity of the particles, and the pres- ence of the entrapped medium--to justify the use of the terms flocculation and coagulation in describing the emulsion behavior. After this brief appraisal of the nomenclature in the case of sols, the various manifesta- tions of emulsion instability, as mentioned earlier, are discussed below. Reversible Instability This simply signifies any manifestation of emulsion instability in which the disperse droplets have not lost their identity and there is a possibility of restoring the emulsion to its original condition. In most cases, particularly in dilute emulsions, the reversibly unstable emulsion can be restored to its original form by simple hand shaking. As men- tioned earlier, creaming and flocculation represent this kind of insta- bility, and these two forms are discussed below. Creaming Creaming is simply caused by the density differences between the disperse phase and the dispersion medium. In the case of true creaming, the emulsion is separated into two emulsions: one is richer in the dis- perse phase and the other poorer than the original emulsion. If the
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)