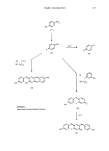
PHYSICAL PROPERTIES OF ALPHA-KERATIN FIBERS 387 I STRUCTURI]CHEMICAL]& MODIFICATION (PHYSICAL , (ELECTRON, X-RAY)J , i CALORI ( ST.UCTUA OR• - _PROPERTIES DY• E LEC TRON M EAS URE MENTS MICROSCOPY DIELECTR IC• CONDUCT I0 N ) PLASTICISERS [WATER• ALCOHOLS• ETC.) RY Figure 1. A diagrammatic representation of the typical physical measurements employed in obtaining the data relating the structure of a fiber to its mechanical properties. techniques, such as reduction of disulphide content of a fiber or swelling in concentrated aqueous lithium bromide solution, can also be assessed in terms of the resultant change in the dynamic and equilibrium properties of the fiber. Thus, the reviewer proposes to examine the mechanical properties of c•-keratin fibers interpreted in conjunction with data obtained with a range of physical techniques. A general introduction has been given to the fibrous protein nature of c•-keratins without any indication to the morphology of such fibers, the distribution, size, and role of various components forming such fibers. Obviously the latter factors play their role in the mechanical properties of fibers and a brief discussion follows on this morphology as it relates to hairs, fur and wools. MORPHOLOGY Alpha keratin fibers such as hairs, furs, and wools have in common the structural components of cuticle and cortex with a central medulla often present in the coarser fibers. The cuticle covers the whole fiber and consists of layers of scales each about half a micron thick, overlapping to give a ratchet-like profile. The number of scales present in a cross-section of a fiber is dependent on the type of fiber. In human hair these may be as many as ten overlapping scales in one cross-section in a newly formed fiber, whereas in a wool fiber only sufficient scales for barely overlapping the fiber exist. The
388 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ratchet-like effect of the scales of the cuticle give the fibers the directional friction effect and play a vital role in the entanglement of the fibers (5). The mechanical action of disentangling as in combing and brushing of hair also tends to strip the cuticle structure. Microscopic examination of human hair shows a progressive removal of the scale structure from root to tip with a complete removal of scale structure being associated with the splitting and fracture of the hair at the tip end (6). In the undamaged hair a very thin membrane (about 3nm thick), the epicuticle, surrounds the outer surface of the fiber. Beneath this is the exocuticle, a cystine-rich component of each scale cell representing about two-thirds of the cuticle structure. The remainder of the scale structure is the endocuticle, followed by a thin layer of cell membrane complex. The endocuticle is the mechanically weakest part of the cuticle and as a whole has a low cystine content. For futher detail of the cuticle structure the reader is referred to the recent report of R.D.B. Fraser et al. (7). Physically the material forming the cuticle does not display any degree of molecular ordering as indicated by such simple measure- ments as optical birefringence (8). The cuticle does have a mechanical protective role for the rest of the fiber, and acts as an important barrier to dye sorption by the fiber, but in terms of bulk physical properties such as longitudinal mechanical properties, its role is very secondary. Only in torsional mechanical properties, where the outside of the fiber has a major mechanical role, can one differentiate between the mechanical properties of cuticle and that of the bulk of the fiber. Although some torsional measurements for wool fibers in which fibers were examined with their scale structure intact, and their surface structure removed, do exist, because scale structure represents such a minor component of wool fibers, any positive conclusion with regard to cuticular property would be hazardous (9). Far more significant results should be forthcoming from measurements on human hairs, where the cuticular structure represents a far bigger proportion of the fiber. The bulk of an &-keratin fiber is the cortex consisting of elongated cortical cells of irregular cross-section of a few microns, packed tightly together and oriented parallel to the fiber axis. The cortical cells when viewed under an electron microscope are seen to consist of long uniform filaments, referred to as microfibrils, of about 7.Sn.m in diameter and of the order of 10n.m center to center oriented parallel to the fiber axis. The microfibrils are grouped into units of about 0.5 microns diameter and are known as macrofibrils. The micro fibrils are embedded in a cystine rich matrix and within any macrofibril are spatially related to each other by a hexagonally packed or whorl-like configuration as seen in cross-sections under an electron-microscope. Physical evidence on extended samples of keratin fibers suggest that the microfibrils within any one macrofibril extend cooperatively and hence must be closely interacting with each other (10). The microfibrils within the &-keratin structure contain the &-helical material responsi- ble for the characteristic &-helical X-ray pattern (11). These microfibrils do not appear to differ from one form of ce-keratin to another, and the evidence strongly suggests that they are essentially crystalline (12). The dry to wet moisture uptake of a hair or wool fiber results mainly in diametral swelling (•- 16%) with little change in the fiber direction (,-- 1.2%). This results from the water uptake being confined mainly to the matrix with the microfibrils moving laterally apart. Any tendency to swell in the longitudinal direction is opposed by the microfibrils, whose stability in water appears little affected. It is only when the &-helices in the microfibrils are wholly or in part destablized that it is possible to obtain any major longitudinal change with dry-wet swelling (13).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)