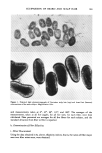
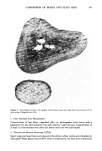
j. Soc. Cosmet. Chem., 34, 351-359 (November 1983) Application of a new microbiological technique to the study of antiperspirant and deodorant soap efficacy R. F. THEILER, C. L. SCHMIT, andJ. R. ROHEIM, Armour Research Center, New Science and Technology Department, 15101 N. Scottsdale Road, Scottsdale, AZ 85260. Received January 10, 1983. Synopsis A new technique for sampling cutaneous microorganisms has been used to study the effects of deodorant products on aerobic axillary microbial populations. Initially the Thran spray gun was compared to the mechanical scrub method and was found to compare favorably in terms of reproducibility and sensitivity. When a commercial stick antiperspirant was evaluated, both methods demonstrated similar log reductions in axillary bacteria when compared to controls. The Thran spray gun was also used to study the efficacy of a commercial deodorant soap containing 3,4,4'-trichlorocarbanilide. A 55% reduction in aerobic axillary microorganisms was demonstrated 24 hours after washing with the soap. INTRODUCTION Many locations on the surface of human skin emit distinctly different odors. However, of all the human scents, those emanating from the axilla are regarded by society as some of the most offensive. In recent years the accumulation of a significant body of scientific evidence has traced the source of axillary odors to the action of bacteria on secretions from the apocrine gland (!-4). In a number of recent publications it has been suggested that a class of Gram positive microorganisms, the diphtheroids, are responsible for the selective generation of the distinctly pungent axillary odors, while the micrococci are responsible for the generation of sweaty, acid odors (2-4). Therefore, numerous manufacturers of deodorant products have marketed formula- tions with an active ingredient which reduces axillary microbial populations for the purpose of reducing the intensity of axillary odor. Since bacterial reduction is related to a product's deodorant efficacy, numerous methods have been developed for qualitatively and quantitatively monitoring the distribution and population of skin flora. Among these are mechanical scrub techniques (5-6), swabbing procedures (7-9), tape stripping and contact plates (7-13), as well as basin scrubbing methods (14-15). Among these techniques, a widely employed procedure for quantitating aerobic skin micro flora has been the mechanical scrub technique which involves a timed scrub of the skin surface with a blunt teflon scrubber 351
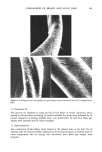
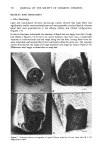
352 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS in the presence of a buffer containing surfactant. Certain problems are encountered with the mechanical scrub procedure, particularly in situations in which a panelist's axillae must be repetitively extracted. In these cases mechanical scrubbing can result in localized edema and irritation of the test site. In the present investigation we describe the use of a more gentle, pressurized spray technique (Thran bacterial sampler) to quantitatively evaluate total aerobic axillary microbial populations (16). The Thran bacterial sampler was compared to the mechanical scrub procedure, and was subsequently employed to demonstrate the efficacy of a solid stick antiperspirant as well as a deodorant soap containing trichlorocarbanilide (TCC). EXPERIMENTAL A. BACTERIAL SAMPLING Bacterial sampling of the axillary vault was performed utilizing both the mechanical scrub procedure of Williamson and Kligman (5) and the pressurized spray gun technique of Thran (16). In the case of the mechanical scrub method, a glass cylinder which circumscribed a 3.8 cm 2 area was firmly placed onto the exposed skin of the axilla, and 3 ml of 0.075 M phosphate buffer pH 7.9 containing 0.1% Triton X-100 was added. The circumscribed skin surface was rubbed with moderate pressure for one minute with a blunted teflon scrubber sampling fluid was removed by aspiration, and the procedure repeated. The two samples containing extracted bacteria were pooled and stored on ice prior to serial dilution. Recoveries for the sampling fluid averaged 90%. The Thran spray gun bacterial sampler was obtained from Dr. Med Vet. Volker Thran, Smetslaan 9, B 1900 Overijse-Maleizen, Brussels, Belgium. In principle, the Thran spray gun technique involves the use of air pressure to aspirate a sampling solution over an isolated region of the skin surface (see Figure 1--a complete description of the apparatus can be found in reference 16.) A jar containing a 100 mi. aliquot of bacterial sampling fluid consisting of 0.075 M phosphate buffer pH 7.9 containing 0.005% Triton X-100 was attached to the spray gun along with an empty sterile collection jar. To extract bacteria the silicon rubber tip of the spray gun was firmly pressed against the axillary vault, and the sampling solution was simultaneously sprayed onto and aspirated from a 1.77 cm 2 area. With a compressed air pressure of 1.8 bars, aspiration times averaged 65 seconds and sampling fluid recovery averaged 98% for the 100 ml sample. Following extraction, bacterial collection jars were removed from the spray gun and stored on ice prior to serial dilution. The Thran spray gun was sterilized between uses by consecutive rinses with sterile water (15 ml), 3A alcohol (15 ml), and one final sterile water rinse (25 ml). Total aerobic bacterial count was determined by serially diluting I ml aliquots from each sample in 10 fold steps with 0.0375 M phosphate buffer, pH 7.9 containing 0.05% Triton X-100. In deodorant soap and antiperspirant experiments 0.5% lecithin and 5.0% Tween-80 were added to this dilution buffer to neutralize any antimicrobial activity associated with the active ingredients. Diluted samples were plated in duplicate in trypticase soy agar supplemented with 0.25% dextrose, 0.1% yeast extract, and 0.2% Tween-80 to facilitate growth of diphtheroids (17). Following a 48 hour aerobic
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)