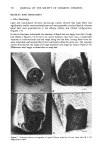
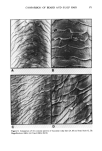
ANTIPERSPIRANT AND DEODORANT SOAP EFFICACY 353 I0 9 4 Figure 1. Schematic drawing of the Thran spray gun bacterial sampler. 1. Compressed air entry 2. Valve 3. Entry to spray head 4. Exit from spray head 5. Air vent 6. Sampling fluid reservoir (detachable) 7. Collecting reservoir (detachable) 8. Special fastening 9. Spray head 10. Sealing band. incubation at 37øC, plates were counted under a dissecting microscope and colony forming units (CFU) per plate converted to log CFU per cm 2 of axilla sampled. Average log CFU's per axilla were calculated for the duplicate plates and paired t-tests conducted when appropriate as a test of statistical significance of differences in axillary aerobic bacterial populations. B. CLINICAL TESTING PROCEDURES Male and female subjects over 18 years of age were recruited for clinical studies. All subjects were in excellent health and free of visible dermatitis. Prior to laboratory testing all iubjects participated in a one week washout period with placebo soap (containing no germicide, fragrance, or color) during which time they were restricted from the use of all deodorant or medicinal soaps, antiperspirants or deodorants, and antibiotics. In addition, subjects were restricted from swimming in chlorinated water within three days of the test period. Female subjects were instructed to stop underarm shaving three days before bacterial extraction. Initial experiments were designed to compare the reproducibility of bacterial extraction using the Thran spray gun or the mechanical scrub procedure. Twenty-four hours after the final placebo soap wash, two adjacent sites on a subject's left and right axillae were extracted--the first site via mechanical scrubbing, the second site using the Thran spray gun. In antiperspirant experiments, following a one week washout period and a placebo soap wash, a commercial antiperspirant stick based on an active ingredient aluminum zirconium tetrachlorohydrex glycine, cyclomethicone, stearyl alcohol, water, glyceryl monostearate, quaternium-18 hectorite, talc, fragrance and SD alcohol 40, was applied
354 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (0.5 gm per male/0.3 gm per female) to either the left or right axilla based on a computer randomized assignment. The corresponding placebo stick, in which the active ingredient was removed and replaced with talc and cyclomethicone, was applied to the opposite axilla. Twenty-four hours later adjacent sites of antiperspirant and placebo treated axillae were extracted, the first site with mechanical scrubbing and the second site with the Thran spray gun. In clinical experiments designed to evaluate the in situ antibacterial efficacy of a commercial deodorant soap based on an active ingredient (3,4,4',Trichlorocarbanilide), sodium tallowate, sodium cocoate, water, glycerin, fragrance, sodium chloride, preservatives, and colors, only the Thran spray gun procedure was employed. Panelists participated in a one week washout with placebo soap (with the same restrictions listed previously) prior to reporting to5 the laboratory. Placebo and deodorant soaps were assigned to opposite axillae using a computer generated randomization schedule, and panelists participated in four supervised washes as described below. Panelists were instructed to wet their right axilla with a clean washcloth (!5 seconds), after which the assigned soap bar was wet under running water (! 5 seconds) and applied directly to the axilla (20 seconds). Following a 15 second hand rinse, panelists wet a clean washcloth and removed all remaining soap lather from their axillae (45 seconds). Panelists' axillae were patted dry with a clean towel, and the procedure was repeated for the opposite axilla with the other test soap using a fresh supply of linen. This process was repeated each morning for four mornings. Twenty-four hours following the last wash each axilla was extracted using the Thran spray gun, and samples were serially diluted for plating and analysis of total aerobic bacterial count as previously described. RESULTS AND DISCUSSION The Thran spray gun (TSG) and mechanical scrub (MS) techniques represent distinctly different methods of bacterial extraction. The TSG delivers a fine mist of buffer and surfactant which we believe removes only surface bacteria. For TSG sampling, the surfactant concentration (Triton X-100) was reduced to 1/20th of the level used with the MS procedure to reduce foaming during extraction. The MS procedure involves extensive agitation of the skin surface resulting in the removal of greater amounts of the desquamating stratum corneum. In contrast to the MS method where microorga- nisms present in lower levels of the stratum corneum might also be removed, the tendency of the TSG is to only remove surface microorganisms. For this reason there was concern about the percentage of total axillary skin bacteria extracted utilizing either method. To answer this question, initial experiments focused on evaluating the in situ axillary bacterial levels on human subjects 24 hours following a supervised wash with placebo soap. For purposes of comparison, adjacent areas in the axillary vault of each panelist were extracted, with the first site using the MS method and the second site using the TSG. This process enabled a comparison of total aerobic bacteria extracted and an examination of the reproducibility of both methods. In addition, total aerobic bacteria could be compared between left and right axillae. Experimental results for five panelists are illustrated in Table I with bacteria levels for each subject expressed in log units per cm 2. Based on the MS method, total axillary bacterial count was found to range from 10 5 tO 10 7 per cm 2 which is in agreement with previously published information (15-19).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)