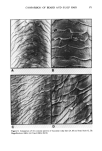
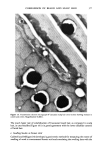
402 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS determination of each release rate. Several other tions as to the selection of these silicone copoly- differences and similarities will be discussed. mers for cosmetic formulations will be discussed. Xanthan gum and its role in personal care products Tashie D. Zang, Kelco, Inc., 8355 Aero Drive, P.O. Box 23076, San Diego, CA 92123 Xanthan gum is the high molecular weight, extra- cellular polysaccharide from Xanthomonas campes- tris. Solutions containing xanthan gum have high viscosities at low gum concentrations, are highly pseudoplastic, and possess a yield value. They also show viscosity which is highly stable in the pres- ence of salt and over wide ranges of pH and temperature. In addition, xanthan gum is compati- ble with many formulation ingredients, such as acids, bases, reducing agents, salts, solvents, enzymes, surfactants, preservatives, and other thick- eners. Some of the benefits provided by xanthan gum in personal care products are (a) good emulsion stabil- ity (b) low viscosity under shear, which allows ease of application (c) excellent suspension of particu- late solids, which is most useful in highly pig- mented products as well as some medicated prod- ucts (d) enhanced foam stability, an important feature in liquid shampoos and liquid soaps (e) good skin feel (f) no "bailing up" of the product on application (g) improved viscosity stability and uniformity with temperature change. Structure/property relationships for silicone polyalkyleneoxide copolymers and their ef- fects on performance in cosmetics M. L. Gum, Ph.D., and S.C. Vick, Ph.D., Union Carbide Corp., Old Saw Mill River Road, Tarry- town, NY 10591 High performance, silicone based wetting agents, commonly referred to as silicone polyalkyleneoxide copolyols, are frequently used in the personal care and cosmetic industries. The unique, highly designed structures of commercially available sili- cone polyalkyleneoxide copolymers impart proper- ties desirable to the cosmetic formulator. These versatile additives find utility as emulsifiers, moisture barriers, flow control and leveling agents, softeners, dispersants, lubricants, potential wetting agents, etc. Utilizing model compounds, the effects of structural changes (such as molecular weight, degree of organomodification, silicone/poly- alkyleneoxide balance, or type of polyether side chain) on the various wetting properties were assessed. Differences in surface tension lowering, cloud point, wet out times, and foam heights were related to structural modifications. Some sugges- Free amino acids of stratum corneum as a biochemical marker to evaluate dry skin J. Koyama, I. Horii, K. Kawasaki, Y. Nakayama, Y. Morikawa, and T. Mitsui, Ph.D., Shiseido Labora- tories, 1050 Nippa-cho, Kohoku-ku, Yokohama, Japan 223 Free amino acids of stratum corneum were studied by using simple extracts from facial skin surface of female subjects. The composition of free amino acids obtained from dry and flaky skin (dry skin) was significantly different from those of normal skin, in which citrulline, alanine, pyroildone carboxylic acid, and urocanic acid decreased, and their precursor amino acids increased. These changes were found to be similar to those observed in hyperkeratonic skin induced by an application of detergent. Our previous findings indicated that free amino acids and their metabolites from stratum corneum are produced via degradation of certain epidermal proteins and further metabolization during the final stage of the keratinizing process. These results suggest that the hyperproliferative alteration of epidermis is involved in dry skin during winter months. The present study indicates that the analysis of free amino acids extracted from skin surface will provide a non-invasive biochemi- cal method for evaluating dry skin conditions. Organoclays in cosmetics Thomas W. Powell, Jr., Clays and Minerals Divi- sion, United Catalysts Inc., P.O. Box 32370, Louis- ville, KY 40232 The function of organoclay in cosmetics will be described in detail, as well as the mechanism of gel formation. The effect of the solvency of various organic fluids on gel properties will also be dis- cussed. The important role of moisture in gel formation will be stressed, with nail lacquer systems as an example. Practical hints for quality control of organoclays will be given that can directly relate to product performance, such as suspension of active materials in antiperspirants. Electron micrographs of various organoclay materials help visualize per- formance properties. Utilizing these concepts, the cosmetic formulator should be better able to pre- dict how organoclay products will perform in a given system and how to solve production prob- lems with organoclays.
ABSTRACTS 403 Free formaldehyde in anionic shampoos Marvin Rosen, Ph.D., and Andrew McFarland, Ph.D., Glyco Inc., P.O. Box 3187, Williamsport, PA 17701 An analytical method has been developed for determining the free formaldehyde content of anionic shampoos, with and without protein, with formaldehyde derived preservatives. The preserva- tives studied were 1,3-dimethylol-5, 5-dimethylhy- dantoin, methane bis [N,N'-(5-ureido-2, 4-diketo- tetrahydroimidazole)-N,N'-dimethylol], N-(hydrox- ymethyl)-N-(1,3-dihydroxymethyl-2, 5-dioxo-4-imi- dazolidinyl)-N'-(hydroxymethyl) urea, cis-l-(3-chlo- roallyl)-3,5,7-triaza- 1-azoniaadamantane chloride, and formaldehyde. The method is based upon establishing an equilib- rium of formaldehyde between the sample, the vapor phase, and an aqueous trapping solution. The trapping solution is measured colorimetrically for free formaldehyde at 513 nanometers using phenyl- hydrazine hydrochloride. Studies were conducted at temperatures of 23øC and 60øC at concentrations of 0.1-0.8% contained preservative. The order of formaldehyde release found was methane his [N,N'-(5-ureido-2, 4-diketotetra- hydroimidazole)-N,N'-dimethylol] 1,3-dimeth- ylol-5, 5-dimethylhydantoin N-(hydroxymethyl)- N-(1,3-dihydroxymethyl-2, 5-dioxo-4-imidazolidi- nyl)-N'-(hydroxymethyl) urea cis-l-(3-chloroal- lyl)-3,5,7-triaza-l-azoniaadamantane chloride. The amount of free formaldehyde released was the same at 23 ø and 60øC. Protein reduced the concentration of free formaldehyde present. In the absence of protein, the ratio of free to total formaldehyde increased with decreasing preserva- tive concentration. SCIENTIFIC SESSION III FORMULATING COSMETIC AEROSOLS IN THE 1980'S Aerosol hair care preparations Morris J. Root, Morris J. Root Associates, 900 Green Bay Road, Highland Park, IL 60035 The advent of the aerosol package stimulated the development of not only new products, but new raw materials for use in them. In particular, aerosol hair sprays spawned a host of synthetic resins to replace and supplement the now almost obsolete shellac originally used. As a result of this develop- ment, there are now in the aerosol spray hair care category not only hair fixative sprays, but lusteriz- ers, conditioners, and setting lotions. In the aerosol foam hair care grouping are waving lotions, hair dyes, shampoos, conditioners, and setting lotions. One must also mention aerosol foam depilatories, which might be labelled a "hair control" rather than a "hair care" product. These aerosol preparations are not used solely by women, but have been adopted by men as well. This is especially true of hair fixative sprays which have, to some extent, replaced men's hair dressings packaged in bottles and tubes. The most recent development is the introduction of the "conditioner and styling mousses" which promise to become another major hair care prod- uct. Fragrance in aerosol products Montfort A. Johnsen, Ph.D., Peterson/Puritan, Inc., Hegeler Lane, Danville, IL 61832 Aerosol products often contain water ranging in pH from 3 to 13. Others are hydroalcoholic, and sometimes contain active ingredients such as phe- nolic or quaternary derivatives. Using the wrong perfume with any of these compositions may cause container corrosion, the development of strange odors and colors, or other unwanted effects. With aerosols containing high levels of isobutane or propane propellants, certain ingredients in the fra- grance blend may slowly precipitate. Some prod- ucts require fragrance to cover chemical odors of other ingredients, while others are used to mask or destroy chemical odors in the home. The degree of atomization must be considered, since a finely dispersed spray will cause significant portions of all the perfume notes to be presented to the nose at the same time, unlike the non-aerosol preparations, where the essential oils evaporate in successive stages. Finally, the amount of fragrance in aerosols ranges from about 0.03% in the "unscented" prod- ucts, past an average value of about 0.18% to as high as 60% for one unusual item. At high levels fragrance oils can cause problems with valve gas- kets. Careful research is needed when developing aerosols containing any amount of fragrance ingre- dients. New era in aerosol propellants John J. Daly, Ph.D., Freon Products Division, Du Pont Company, Chestnut Run, Wilmington, DE 19898 A new line of aerosol propellants has recently been introduced. The four products include: (1) dimethyl ether (2) chlorodifiuoromethane (3) 1,1-difiuoro- ethane and (4) 1,1,1-chlorodifiuoreoethane. The physical, chemical, and toxicological properties of these propellants will be described. Interrelation-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)