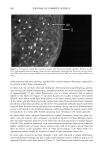
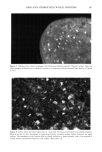
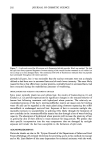
170 JOURNAL OF COSMETIC SCIENCE Exposure of hair to light causes chemical and physical degradation of the fiber (4,5). Recent reports on the harmful effects of sunlight on human skin have raised awareness of the deleterious effects of sunlight on biological tissues in general. Because hair does not have the repair mechanism of skin (6), the effects of photodegradation must be dealt with through cosmetic means. The way that light affects hair is still not completely understood. The solar spectrum's UV range is known to lead to photooxidation of the protein structure through the oxidation of specific amino acids, in particular the oxi- dation of cystine to cysteic acid (7-9). The deleterious effects of solar irradiation are perceived by consumers as changes in texture and color, dryness, etc, and can be evalu- ated in terms of reduced elasticity, increased porosity or swelling properties, altered dye sorption characteristics, and photofading of natural or artificial hair color (10-12). The essential first event in hair photodamage, as in all photoprocesses, is light absorption by the fiber. Free radicals have been observed by electron spin resonance (ESR) spec- trometry during the light-induced degradation of keratin ('13-15). Carbon-centered radicals are formed with an action spectrum maximum of 285 nm, suggesting that they are primary photoproducts of aromatic amino acids (16). Kinetic measurements indicate that there are at least three types of radicals produced at this wavelength (17). Stable sulfur-centered radicals are formed from irradiation of dry keratin in the absence of oxygen, but their ESR signals disappear in the presence of moist air (18). The presence of small amounts of metal ion (trivalent iron or divalent copper) is especially conducive to the production of free radicals from irradiated keratin at wavelengths above 320 nm, apparently due to the formation of metal-protein complexes that absorb at higher wavelengths (19). Smith et aL (20) have suggested the presence of tetrahedral iron-sulfur centers that are easily oxidized upon irradiation, giving rise to carbon-centered radicals on neighboring groups. The most significant chromophores in proteins that absorb in the UVB region are the amino acids, tyrosine (Tyr, •max = 275 nm) and tryptophan (trp, •max = 280 nm). The irradiation of wool keratin under UVA and visible light does not cause the formation of tryptamine, which is the main photoproduct formed during irradiation of tryptophan itself in aqueous solution (21,22). Like wool photoyellowing and photodamage, a free-radical mechanism may be involved in the photodamage of hair at wavelengths above 320 nm. Melanin has an intrinsic ESR signal that increases significantly when irradiated with UV-visible light. In the presence of oxygen, superoxide is produced, which dismutates to hydrogen peroxide (23,24). This leads to the formation of hydroxyl radical in the presence of trace amounts of metal ions. We have recently extended the observations of earlier workers to the study of oxygen radical production in suspensions of melanin extracted from red and brown hair (25). By employing terephthalic acid dianion (TA) as a hydroxyl radical trap, we have introduced a convenient method for estimating relative amounts of oxyradicals formed under varying conditions. In the terephthalate analysis, TA reacts with hydroxyl radicals to form hydroxyterephthalate (HTA), as shown in Scheme I (26). HTA is highly fluorescent, with an excitation wavelength sufficiently high that there is little or no interference from tryptophan fluorescence. Our results on the melanin suspensions show that while red hair melanin (largely pheomelanin) has a smaller intrinsic ESR signal, and a smaller increase upon irradiation, compared to brown hair melanin (largely eumelanin), irradiation of red hair melanin produces a larger yield of oxygen radicals than either brown hair melanin or sepia, which is pure eumelanin. In this paper, we report on the combined use of ESR spectroscopy
OXYRADICALS FROM PHOTOIRRADIATED HAIR 171 oo + OH- -- r r OO- H COO- Terephthalate (TA) (Non-fluorescent) 2-hydroxyterephthalate (HTA) (Fluorescent: 3.ex = 315 nm, •'em -- 425 nm) Scheme 1 and reaction with TA to demonstrate and assess the involvement of oxygen radicals when hair is irradiated at wavelengths above 320 nm. EXPERIMENTAL MATERIALS All chemicals were reagent grade quality and were used without further purification. Terephthalic acid, sodium hydroxide, and potassium phosphate monobasic (KH2PO4) were purchased from Fisher Scientific Company (Fair Lawn, NJ). DMPO (5,5-dimethyl- 1-pyrroline N-oxide), 2-bromoterephthalate, and sodium azide were purchased from Sigma (St. Louis, MO). Sodium benzoate, and sodium phosphate dibasic (Na2HPO 4 ß 7H:O) were from Allied Chemical (New York). Ethanol (200-proof) was from Quantum Chemical (Tuscola, IL). Standard 2-hydroxyterephthalate (HTA) was synthesized from 2-bromoterephthalate by the method of Mason et al. (27). The crude precipitate was purified using liquid column chromatography until the fluorescence spectrum matched that from the literature (28). The melting point of the purified crystals is 321-325øC (lit. m.p. 320-322øC). This authentic HTA was used for the standard curve. For the HTA calibration curve, a 2.0 mM HTA (pH 7.6) stock solution was diluted with 50 mM phosphate buffer (pH 7.6). The standard curve was obtained by plotting fluo- rescence intensity at 425 nm against HTA concentration (25) it is linear up to at least 10 pM. The milled keratin powder was provided by Zotos Corporation. Hair tresses were pur- chased from DeMeo Brothers. Before each experiment, the hair samples were washed with 4.5 % sodium lauryl sulfate and doubly distilled water, and left at room tempera- ture to dry. The clean and dry hair sample was cut into 1-mm-long pieces. The cut hair or wool powder was added to a 1- or 2-ml buffered solution of ESR spin trap (DMPO) or fluorescence probe (TA) in a 1-cm square Pyrex spectrometer cuvette for irradiation. Samples were normally stirred to maintain air saturation.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)