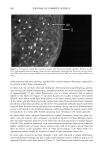
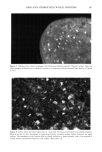
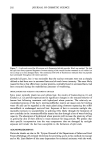
196 JOURNAL OF COSMETIC SCIENCE 30 25 ß • 20 • lO 5 0 20 30 40 50 I Tkne (minutes) Figure 1A 2.5 2 1.5 0.5 0 -0.5 10 20 30 Tkne (m•utes) Figure lB 1.8 1.6 1.4 1.2 0.8 0.6 0.4 0.2 0.0 -0.2 0 10 20 30 40 5o T•e (mhutes) o lO 20 3O Tkne (minutes) Figure 1D Figure 1C Figure 1. Size-exclusion HPLC chromatograms. A. Initial hydrolyzed wheat protein B. FITC control. C. FITC-labeled peptides before purification. D. Purified FITC-labeled peptides. 1. Untreated root-ends--brown Caucasian. 2. Split ends--blonde Caucasian. 3. Bleached--root-end brown Caucasian (using commercially available bleaching kit [Wella Hair Streaking Kit] two treatments given). 4. Permanently waved--root-end brown Caucasian (using commercially available perm treatment [Alberto VO5 select perre] two treatments given). 5. Relaxer treated--root-end brown Caucasian (hair relaxer RS-4-30-1 [formulated by Croda Oleochemicals] containing 2% sodium hydroxide used as a single treatment). 6. Relaxer treated--ethnic black (as in 5, but hair was physically straightened during treatment). Hair types were supplied by De Meo Brothers, New York, and International Hair Importers and Products Inc., New York. Swatches (made up of 30 x 4 cm hair fibers) of each of the above were treated with aqueous solutions of the fluorescently labeled hydrolyzed wheat protein for 30 minutes or overnight (16 hours), rinsed (in 2 x 250 ml water, each rinse lasting 10 seconds),
HAIR AND HYDROLYZED WHEAT PROTEINS 197 blotted dry between tissue paper, and dried overnight in a desiccator over silica gel. Fibers from each sample were individually stretched between slits in small silicone molds, acrylic embedding resin "LR White" (London Resin Co., Reading, England) was poured in, and the fibers polymerized at 60øC for 24 hours. Two slightly different methods were used for preparing specimens for the microscope. In one case transverse sections of 25-ylm thickness were cut from each block on a steel knife, using a regular histology microtome, and these were laid onto appropriately labeled individual micro- scope slides. In the other case, the resin block used for the preparation of the sections was trimmed further to define an area of about 300-1•m square around each fiber. This transverse surface was then smoothly faced using a freshly prepared glass knife on a Reichert UM2 ultramicrotome to pare off sections of gradually decreasing thickness down to 50 nm. Each resin block (of ca. 10 mm height) was glued to a microscope slide with the smoothed face uppermost and, as near as could be judged by the eye, parallel with the plane of the slide. All the microscopy work was carried out using a Biorad MRC600 laser-scanning con- focal attachment to a Nikon Optiphot light microscope. This provided the capability not only for confocal laser fluorescence imaging but also for transmission imaging with tungsten or UV light sources and a selection of different microscope objective lenses. The section and block tops were examined initially at low power under tungsten illumination and sometimes using UV. Once a hair had been located, it was centered in the field, a drop of immersion oil was added, and then the hair was imaged in the laser-scanning confocal mode at higher magnifications using a x60 1.4 NA oil-immersion objective. The specimen was scanned with a beam from an argon-ion laser (•kma x = 488 rim, spot size 0.5 l•m), and the system was tuned to provide images exclusively from the fluo- rescent emissions of the fluorescein-labeled peptides (at h. -- 518 nm) within an optical slice of the specimen between 0.5 and 1.0 lnm thick. The vertical position of the specimen was adjusted so that optical slicing was just below the upper surface of the sections and of the smoothed block tops. Image magnification was varied according to changes in the overall dimension of the laser-scanned field. Images were averaged by Kalman filtering (30 frames) to improve their signal-to-noise content and stored on computer disk. RESULTS AND DISCUSSION The various types of hair were treated with the fluorescent probe specifically attached to the target peptides and free from either unreacted FITC or FITC decomposition products as demonstrated by size-exclusion HPLC analysis. Since the peptides contain only 1.46 mole% of lysine, most of the fluorescein level will be covalently attached to the end- amino groups of the peptides. A major concern was that the addition of the fluorescent probe to the hydrolyzed wheat proteins should not affect their diffusion behavior in hair. The molecular mass of each peptide fragment will have increased by 389 Da in its reaction with FITC, accounting for a 38.9% increase for peptides of average mass (ca. 1000 Da) in the initial mixture. The reaction will also have eliminated one amino function and introduced one new carboxyl function into each peptide unit. The starting peptides already contain 40% (by weight) of their amino acid residues as glutamic and aspartic acids (37% and 3%, respectively), or 36 mole%, and so their anionic character will have been enhanced in the
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)