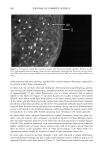
198 JOURNAL OF COSMETIC SCIENCE fluorescein conjugates. Thus for peptides of average molecular mass and containing eight amino acid residues, the effective side-chain carboxyl function will have increased from an average of four carboxyl groups (including the terminal group) per peptide chain to five carboxyl groups per chain. While the original peptides have clearly been altered, the overall changes are not considered to be excessive. We believe therefore that the fluo- rescently labeled material will have provided a realistic model for studying the diffusion of unlabeled wheat protein hydrolysates into hair. Both methods of specimen preparation yielded satisfactory information about the pen- etration of the fluorescently labeled peptides into the various hair types. The confocal images showed the hair's internal structure with a clarity (i.e., resolution) significantly better than is normally provided by the conventional fluorescence microscope. It was possible, for example, to identify the hair's endocuticle by the irregularity of its outer- facing surface and the smoothness of the inner-facing surface. Both methods for pre- senting specimens to the microscope, yielded similar information. The hairs in the physical sections were often torn (Figure 2), whereas the smoothly planed block tops were free from such imperfections (Figure 3) and were thereby a preferred method of hair sample presentation. All samples (untreated ones and those pretreated by various cosmetic processes) con- tained the labeled peptides in discrete structural locations throughout the entire cross section of each hair (Figures 2-7). For the undamaged root-end hairs (Figure 2), lesser amounts of fluorescer were contained within samples treated with the peptides for 30 minutes than in those treated overnight (Figure 3). In both, the highest levels occurred in the cuticle and peripheral cortex, and from there the intensity fell off rapidly inwards. Figure 2. Root-end hair treated with fluorescein-labeled peptides for 30 minutes. Physical section. Fluo- rescence throughout the hair, but higher concentrations at its periphery. Arrows indicate tear in the section (T).
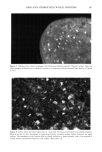
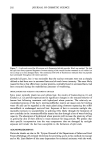
HAIR AND HYDROLYZED WHEAT PROTEINS 199 Figure 3. Root-end hair treated with fluorescein-labeled peptides overnight. Block top. Fluorescence throughout the hair, but higher concentrations at its periphery and a greater overall level of fluorescence compared with the 30-minute treatment shown in Figure 2. One therefore adduces that, even after overnight treatment, not all the potential sites of occupation for the peptides are occupied. The highest intensity of fluorescence occurred at the perimeter of the undamaged root-end hairs, indicating that water rinsing had not removed significant amounts of material. In all samples the fluorescent peptides were contained principally within the endocuticle and in the nuclear remnants and intermacrofibrillar matrix of the cortex (Figures 4, 6). The boundaries of the cortical cells were also highlighted. The limited resolution of the images did not enable us to define the precise location of the peptides at the cell boundaries. On the other hand, we believe they are more likely to be contained within the non-keratin peripheral intracellular envelope of each cortical cell than within the adjacent cortical cell membrane complex (5). The nuclear remnants of the cortex pre- sented strikingly high levels of fluorescence that highlighted their characteristic stellate shape wherever they occurred within the transverse fiber cross sections. Hairs not treated with the labeled peptides were completely free from fluorescence at the detected wave- length (518 nm). That low levels of fluorescence were found in the exocuticle and cortical macrofibrils of peptide-treated hairs attests to penetration even of these dense structures. The main sites of residence for the peptides in the hairs are consistent with these same structures also acting as the principal pathways for the diffusion of aqueous-borne materials into human hair (5). Despite their average molecular mass in excess of 1000 Da, the peptides are clearly capable of penetrating the full depth of the human hair shaft. The high concentrations found in the nuclear remnants of the cortex and in the endo- cuticle are probably brought about by ionic interaction between the anionic hydrolyzed
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)