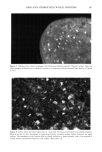
172 JOURNAL OF COSMETIC SCIENCE All irradiation was done with a 150-W xenon lamp through glass filters transmitting UVA and visible light (--320 nm). The measured light flux falling on 50% of a 2-ml sample was typically 0.35 mw/cm 2 (+ 15%). After irradiation, each sample was filtered through a 0.22-}am filter, and the flitrate was transferred to an ESR quartz fiat cell for ESR measurements or to a fluorescence cell for fluorescence measurements. To minimize the effects of varying lamp intensity and other factors, series of experiments comparing different samples were always done on the same day. Replicate experiments performed on different days were generally within + 10% for HTA or +20% for ESR experiments, but relative trends for different types of hair were always the same. INSTRUMENTAL Electron spin resonance (ESR) measurements were performed at room temperature on a Bruker ER200 spectrometer operating at 9.76 GHZ with 100 KHZ field modulation and microwave power of 20 mW. Direct ESR spectra of hair were taken in 3-mm quartz tubes. For the spin trapping experiments, irradiation (in 1-cm cuvettes) was done outside of the ESR cavity, and samples were transferred to the fiat cell after irradiation. While irradiation of melanin within the ESR cavity permitted detection of the superoxide radical (25), the instability of DMPO-O 2- allows only DMPO-OH to be observed in the present experiments. Fluorescence spectra were taken with a Spex Fluorolog-1680 spectrometer in 1-cm disposable plastic cells. HTA signals (kex = 315 nm, )kem= 425 nm) were identified and quantified by comparison with synthetic HTA. All fluorescence measurements reported here are on the same arbitrary scale, with a value of 10 corresponding to 0.1 }aM of HTA. Because of absorption, fluorescence, and/or fluorescence quenching, individual reference solutions were prepared for each experiment containing the same concentrations of all components except hair (or keratin). Irradiated solutions of HA in the absence of hair or other additives did not give significant production of HTA. RESULTS AND DISCUSSION The intrinsic ESR signal for a variety of hair types, as well as for milled wool keratin, is shown in Figure 1. The pigmented black, brown, and red hair exhibits comparatively large signals (g = 2.004), due essentially to the presence of melanin. The broader and much weaker signals from blond, white, and bleached hair have a slightly lower g value and are similar to those of milled keratin. Figure 2 shows the buildup and decay of melanin signals from brown and red hair. The increase of the intrinsic melanin signal under irradiation for the red hair is smaller than that for the brown hair, a difference which we also noted with suspensions of extracted melanins (25). Figure 3 demonstrates that when wool keratin is irradiated with UVA and visible light in the presence of terephthalate ion, HTA is formed, indicating that hydroxyl radicals are produced during this process. The production of HTA is quenched when ethanol or sodium azide is added to the reaction mixture. Both ethanol and sodium azide are hydroxyl radical scavengers, and thus compete with TA for the hydroxyl radicals produced when the keratin is irradiated. Human hair has a structure similar to that of wool (5). When hair is irradiated in the presence of TA, HTA is also produced. Figure 4 compares the yield of HTA from different varieties of hair after irradiation for 90 minutes. As in our previous study (25), we observe a steady increase in HTA with increasing irradiation
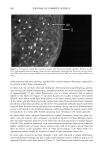
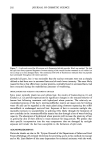
OXYRADICALS FROM PHOTOIRRADIATED HAIR 173 Keratin x8• • White H air x80 • _•..••'•-•• Bleached Hair x80 10G Black Hair xl Red Hair x5 Brown Hair xl Figure 1. Intrinsic ESR signals for milled wool keratin and human hair. Spectrometer settings: modulation amplitude, 4G receiver gain, 1.25 x 104 microwave power, 20 row. Each was 0.03 g in a 2-ram I.D. quartz tube. time. Figure 5 shows the effects of azide and ethanol on HTA production from irradiated bleached hair. ESR spectra are displayed in Figure 6 for the trapped radical adduct from bleached, red, and brown hair irradiated in the presence of DMPO. The spectra are characterized by hyperfine coupling constants of a N = 14.9 G, aH • = 14.9 G, showing that the radical is the DMPO-OH adduct (29,30), and confirming that oxyradicals are produced during irradiation. For both the fluorescence and the ESR experiments, bleached hair and red hair give much larger signals than brown hair. When cinnamic acid is present, no DMPO-OH adduct is observed. Figure 7 gives the result from red hair other hair types show similar results. Since at the wavelengths (k -- 320 nm) and concentrations of our experiments, cinnamic acid does not have a significant absorption, it seems also to be competing for oxyradicals. This is not unex- pected given its aromatic ring and unsaturated side chain. (We note that parallel fluorescence measurements could not be performed because of high background fluo- rescence of cinnamic acid.)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)