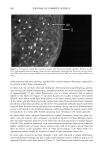
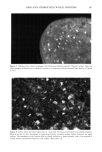
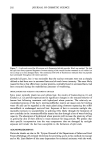
194 JOURNAL OF COSMETIC SCIENCE during the drying of wet hair is supported by recent dynamic vapor sorption experiments by Chahal et al. (2). Interestingly both Gamez-Garcia (1) and Chahal et al. (2) found there was no significant difference in the equilibrium moisture regain between untreated hairs and those pretreated with the hydrolyzed protein, the only effect being on the rate of approach to moisture equilibrium over a range of relative humidities. As an aid to explaining these effects we now report experiments designed to find which parts of the hair structure absorb the hydrolyzed wheat proteins. Novel radiolabeling experiments by Jones and Chahal (3) have provided the means for discriminating be- tween hydrolyzed wheat proteins at the surface and within the bulk of treated hairs. Their results showed that a significant proportion of the peptides were contained within the hair's bulk but did not reveal whether these were evenly distributed throughout the hair's cross section or restricted just to the hair's cuticle. Nevertheless, the influence on the mechanical behavior of hair in stretching mode suggests penetration into the cortex. Experimentally it is extraordinarily difficult to detect the microscopic distribution of small amounts of one substance (peptides) inside another of similar chemical composi- tion (human hair). One requires an imaging process (of good resolution in the present case) that will signify the presence of the minor component with a high level of specificity. To this end we have chosen to covalently attach a fluorescent label to our peptides and then examine their distribution in treated hairs by means of laser-scanning fluorescence confocal microscopy. The advantages of using this particular microscope were its high specificity of selection for the fluorescent emissions of the label, its capability for "optical" sectioning, and a better resolution by a factor of 2 than the conventional fluorescence microscope (4). MATERIALS AND METHODS Hydrolyzed wheat protein was fluorescently labeled with fluorescein isothiocyanate (FITC), purified to remove any fluorescent moieties not attached to peptide amino groups, and then used to treat samples of treated and untreated human hair: N=C=S + H2N-Pe ptide H-N-C-N-Pe ptide II I s H Fluorescein Thiourea is oth iocyanate d e rivative (FITC) PREPARATION AND PURIFICATION OF THE FLUORESCENTLY LABELED HYDROLYZED WHEAT PROTEIN Fresh fluorescein isothiocynate (Molecular Probes Europe BV, The Netherlands) (10 mg) was dissolved in anhydrous dimethylformamide (1 ml Analgar grade). This solution was
HAIR AND HYDROLYZED WHEAT PROTEINS 195 added to a stirred solution of the hydrolyzed wheat protein (20 ml 10% solution 2.06% active) at pH 7.5 over a period of 30 minutes at ambient temperature. The reaction mixture was stirred for 1 hour. Hydroxylamine (1 ml 1.5 M pH 8.5 Analar grade) was then added and stirring was continued for a further one hour. This solution was then purified using Sephadex G15 resin (Sigma-Aldrich) gel filtration media. The fluores- cently labeled hydrolyzed wheat protein to be purified was placed onto a column of the Sephadex G15 resin (1000 mm x 25 ram), previously equilibrated with phosphate buffer (0.05 M pH 7.0 containing 0.1 M sodium chloride), and eluted through the column using the same phosphate buffer (300 ml) at a flow rate of 1 ml/min. Fractions (10 ml) were collected and analyzed using size-exclusion HPLC (HP 1100 Tosohaas TSK G2000SWxl column, phosphate buffer eluent [0.05 M pH 7.0 containing 0.1 M sodium chloride], 25øC, UV detection at 220 nm, 0.6 ml/min flow rate). The fractions containing the purified fiuorescently labeled hydrolyzed wheat protein were combined and concentrated by rotary evaporation to approximately 10% total solids and subse- quently analyzed by size-exclusion HPLC to ensure that purity was maintained. ANALYSIS OF THE FLUORESCENTLY LABELED HYDROLYZED WHEAT PROTEIN SOLUTION It was essential that, prior to treating hair, the fiuorescently labeled hydrolyzed wheat protein was pure and free from any contamination with unreacted FITC or FITC de- composition products. Accordingly, a number of experiments and analyses were carried out to confirm that the Sephadex G15 gel filtration resin was capable of effectively separating the various components of the reaction mix. Size-exclusion HPLC (as de- scribed above) was used to characterize the various components of the reaction mixture. In particular, it was used to establish the purity of the fluorescently labeled hydrolyzed wheat protein eluted from the Sephadex column. The following solutions were exam- ined: A. Hydrolyzed wheat protein. B. Control FITC (FITC put through the above reaction procedure, but with the protein replaced by water). C. FITC-hydrolyzed wheat protein reaction mix prior to purification. D. Purified fluorescently labeled hydrolyzed wheat protein. The results in Figure i show that the hydrolyzed wheat protein (A) and the fiuorescently labeled hydrolyzed wheat protein (C,D) both have a peak maximum at an elution time of approximately 20 minutes. The unreacted FITC (B) has a peak maximum at an elution time of approximately 35 minutes. The polydispersity of the chromatograms is low, and the elution times are sufficiently different, such that it is possible to differentiate the various fractions obtained from the Sephadex column during purification. The relevant fractions were collected and the pure fiuorescently labeled hydrolyzed wheat protein was separated from the reaction mix as shown in Figure 1D. The purified fluorescently labeled hydrolyzed wheat protein was analyzed and found to contain total solids (9.3%), ash (7.6%) and thus, by difference, an active labeled hy- drolyzed wheat protein content of 1.7%. It was diluted to 1% active content before being used to treat hair. The following types of hair were chosen for treatment with the purified FITC-labeled peptides:
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)