192 JOURNAL OF COSMETIC SCIENCE (2) I. C. Watt, Sorption of water vapor by keratin,J. Macrotool. Sci. Rev. Macrotool. Chem., C18(2), 169-245 (1980). (3) B. Forslind, in Hair and Hair Disease, C. E. Orfanos and R. Happle, Eds. (Springer-Verlag, New York, Berlin, Heidelberg, 1980), pp. 73-97. (4) J. D. Leeder and I. C. Watt, The role of amino groups in water absorption by keratin. J. Phys. Chem., 69, 3280 (1965). (5) L.J. Pauling, The adsorption of water by proteins, J. Amer. Chem. Soc., 67, 555 (1945). (6) S.J. Smith, The sorption of water vapor by high polymers, J. Amer. Chem. Sot., 69, 646 (1947). (7) P.R. Crippa, V. Cristofoleti, and N. Romeo, A band model for melanin deduced from optical absorption and photoconductivity experiments, Blochim. Biophys. Acta., 538, 164-170 (1978). (8) P. L. Walling and J. M. Dabhey, Moisture in skin by near-infrared reflectance spectroscopy, J. Soc. Cosmet. Chem., 40, 151-171 (1989). (9) K. Martin, Direct measurement of moisture in skin by NIR spectroscopy, J. Soc. Cosmet. Chem., 44, 249-261 (1993). (10) K. Martin, In vivo measurements of water in skin by near-infrared reflectance, Appl. Spectroscopy, 52(7), 1001-1007 (1998). (11) J. deRigal, M. J. Losch, R. Bazin, C. Camus, C. Sturelle, V. Descamps, and J. Leveque, Near-infrared spectroscopy: A new approach to the characterization of dry skin, J. Soc. Cosmet. Chem., 44, 197-209 (1993). (12) Y. Ozaki, T. Miura, K. Sakurai, and T. Matsunaga, Nondestructive analysis of water structure and content in animal tissues by FT-NIR spectroscopy with light-fiber optics. Part I: Human hair, AppL Spectroscopy, 46(5), 875-878 (1992).
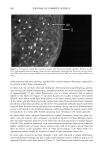
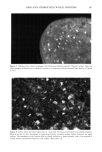
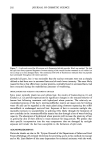
Cosmet. Sd., 51, 193-203 (May/June 2000) Investigations on the penetration of hydrolyzed wheat proteins into human hair by confocal laser-scanning fluorescence microscopy J. A. SWIFT, Department of Textile Design & Prodution, De Montfort University, The Gateway, Leicester, LE1 9BH, England and S. P. CHAHAL, N. I. CHALLONER, and J. E. PARFREY, Croda Colloids Ltd., Foundry Lane, Ditton, Widnes, Cheshire, WAS SUB, England. Accepted for publication March 15, 2000. Synopsis Hydrolyzed wheat proteins, of a type commonly used in hair toiletry products, were labeled by reaction with fluorescein isothiocyanate. Aqueous solutions were applied to untreated hair and also to hairs treated beforehand by permanent waving, by bleaching, or by relaxers. The extent of penetration of the peptides into the hairs and their structural locations in them were determined by imaging transverse sections of the resin-embedded hairs with a confocal laser-scanning fluorescence microscope. Experimental evidence and arguments are mustered that the fluorescently labeled peptides, having been purified, provide a satisf•tctory model for studying the diffLsional behavior of the native peptides in hair. Penetration of the hydrolyzed wheat proteins into all the hairs was extensive. The main sites for occupation of the peptides were the endocuticle and in the cortex, the nuclear remnants (very intense), intermacrofibrillar matrix, and along the cell boundaries. INTRODUCTION The absorption of partially hydrolyzed wheat proteins (Cropeptide W TM, hydrolyzed wheat protein supplied by Croda Oleochemicals, Snaith, Goole, England, and Croda Inc., Parsippany, NJ) into human hair from aqueous solution was shown by Gamez- Garcia (1) to alter the tensile mechanical properties of the fibers, notably as they were being dried. He found that hairs rapidly stretched by 1% at constant relative humidity, briefly immersed in water, and then withdrawn to an atmosphere at the same relative humidity, took four minutes to recover 80% of their initial tensile stress. On the other hand, for a similar experiment carried out with a solution of the hydrolyzed wheat protein, recovery to 80% initial stress took many hours. That such a dramatic effect might be due to the ability of hydrolyzed wheat proteins to impede the loss of water Address all communications to Dr. J. A. Swift, 29 Moorland Park, Gayton, Wirral, CH60 8QJ, England. 193
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)