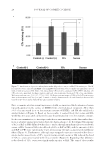
HYDRATION AND SPF TESTING OF LIP BALM FORMULATIONS 11 A and B at 1 h (p 0.0001), 3 h (p 0.0001), and 6 h (p = 0.001 and p 0.0001, respec- tively) post–lip balm application. In addition, test product B demonstrated statistically signifi cant improvement in skin hydration compared with comparator D at 6 h post–lip balm application (p = 0.0064 Table III). The Corneometer data indicated a statistically signifi cant improvement in skin sur- face hydration for test product B compared with all four comparator/reference lip balms (p 0.0009) and untreated dry leg skin (p 0.0001) at all time points postap- plication (Table III). Home-use lip hydration phase. Barrier function and skin hydration data for the four par- ticipants who used test product B during the home-use phase of the study are shown in Figure 1. Mean ± SD TEWL at baseline was 54.60 ± 17.34 g m-2 h and decreased to 43.58 ± 4.61 g m-2 h after 12 d of test product B application, suggesting a slightly reduced level of water loss. The lowest TEWL score occurred at day 8 (mean ± SD 15.34 ± 4.74 g m-2 h). TEWL scores were consistent between the four participants at each time point. Mean ± SD DMM at baseline was 61.00 ± 13.25 arbitrary units (au) and increased to 63.90 ± 27.62 au by day 12, with a maximum score of 88.30 ± 82.34 au on day 8. This small increase suggests that there was no overall change in skin hydration levels. DMM scores were consistent between three of the participants the exception was one participant for whom the DMM score was 210.60 au on day 8. Figure 1. Study RH02116: Home-use lip-hydration phase barrier function and skin hydration data for four participants over the 11-d study period. Scores and mean values for (A) TEWL, (B) DMM, (C) Corneometer, and (D) visual P2 assessment.
JOURNAL OF COSMETIC SCIENCE 12 Mean ± SD Corneometer at baseline was 24.72 ± 3.14 au and decreased slightly by day 12 to 23.22 ± 4.87 au, suggesting no overall change in skin hydration levels. There was a high level of variability in the Corneometer scores for all four participants at all time points. The mean baseline score following visual assessment of lip dryness for the four participants was 4.0. This decreased only marginally to 3.5 by day 12, suggesting no overall impact of the test product on lip dryness. The lowest mean visual score was recorded on day 8 (2.3). SPF determination studies. For all four SPF studies, the SPF standard P2 positive control achieved a mean SPF within the SD range of the intended SPF according to ISO 24444 (16.4 ± 2.4) and FDA fi nal rule (16.3 ± 3.43), thus confi rming the accuracy of the study protocol. The mean value of the tested SPF lip balm formulations did not achieve the intended labeled SPF 15 (Table IV). In study RH01927, there was a single protocol deviation where one participant under- went initial MED evaluation 18 min outside of the permitted evaluation window. How- ever, this did not affect the study results. Study RH02117 did not achieve the number of valid test results required to determine an SPF value for the test products for test product B, only nine acceptable results were obtained, and for test product C, only eight accept- able results were obtained. SAFETY ASSESSMENTS There were no test product-related AEs or SAEs for any of the fi ve lip balm formulations assessed across the fi ve studies. Furthermore, no local skin reactions, such as irritation, due to product application were observed. Table IV Mean SPF Values and Label for Five New Lip Balm Formulations across Four Studies, as per Protocols Defi ned by the FDA Monograph Final Rule (5) or ISO 24444 (6) Study Product identifi cation Regulatory criteria used n (valid cases) Mean SPF ± SD Mean SPF ± CI, % SPF value and label (protection offered) RH01927 Test product A FDA fi nal rule 10 13.7 ± 4.8 10 (low) RH01927 P2 standard FDA fi nal rule 10 14.5 ± 2.3 NAb RH01928 Test product A ISO 24444 13 15.2 ± 3.9 Low RH01928 P2 standard ISO 24444 13 14.8 ± 3.2 NAb RH02117 Test product B FDA fi nal rule 8 13.4 ± 2.4 Invalid dataa RH02117 Test product C FDA fi nal rule 9 13.4 ± 1.5 Invalid dataa RH02117 P2 standard FDA fi nal rule 13 15.4 ± 3.7 NAb RH02385 Test product D ISO 24444 13 13.9 ± 13.9 10 (low) RH02385 P2 standard ISO 24444 13 14.2 ± 12.0 NAb RH02385 Test product E ISO 24444 12 12.8 ± 16.9 10 (low) RH02385 P2 standard ISO 24444 12 13.9 ± 9.9 NAb RH02385 Test product D FDA fi nal rule 10 14.4 ± 12.6 12 (low) RH02385 P2 standard FDA fi nal rule 10 14.4 ± 12.5 NAb RH02385 Test product E FDA fi nal rule 10 15.5 ± 11.4 11 (low) RH02385 P2 standard FDA fi nal rule 10 14.6 ± 12.9 NAb a 10 Subjects achieved valid test results, insuffi cient to determine an SPF value for test products. b Label value not required for standard only test product.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)