J. Cosmet. Sci., 70, 1–15 (January/February 2019) 1 Evaluating the Moisturizing Abilities and Sun Protection Factor of New Lip Balm Formulations CHRISTOPH GFELLER, GEORGE HARDIE, GILBERT SHANGA, and HARISH MAHALINGAM, R&D Medical Affairs Skin Health, GSK Consumer Healthcare, Brentford, Middlesex TW8 9GS, United Kingdom (C.G.), R&D Clinical Operations, GSK Consumer Healthcare, Weybridge, Surrey KT13 0DE, United Kingdom (G.H.), R&D Biostatistics, GSK Consumer Healthcare, Warren, New Jersey (G.S.), R&D Medical Affairs Skin Health, GSK Consumer Healthcare, Warren, New Jersey (H.M.) Accepted for publication September 12, 2018. Synopsis This report explores dry-skin models to assess the potential of a new lip balm formulation to hydrate dry skin or lips, and presents sun protection factor (SPF) values for fi ve new lip balm formulations. Evaporimeter [for transepidermal water loss (TEWL)], Skicon®, and Corneometer® were used to measure hydrating effects of lip balm formulations in a dry-skin leg model, and TEWL, DermaLab® Moisture Meter, Corneometer®, and visual assessments were used with a dry-lip model. SPF studies were conducted in accordance with either the U.S. Food and Drug Administration monograph fi nal rule or international standard ISO 24444. Data from dry- skin leg model demonstrate that a new lip balm formulation signifi cantly improves skin hydration compared with untreated leg skin and four comparator products. Data obtained from a dry-lip model proved unreliable. Five new lip balm formulations exhibited sunscreen capability however, they did not meet the intended SPF. There were no product-related adverse events with the formulations. Although the new lip balm formulation improved hydration, data from a novel dry-lip model proved unreliable therefore further testing is required to confi rm these benefi ts. Five new lip balm formulations provided sunscreen capability but did not meet the intended SPF, and will undergo reformulation and retesting. INTRODUCTION Exposure to small amounts of ultraviolet (UV) radiation is known to be benefi cial, although prolonged UV exposure may be damaging for the skin, eyes, and immune system (1). Erythema, commonly referred to as sunburn, is an acute damaging effect of UV exposure on the skin (2). Prolonged UV exposure, however, may cause degenerative changes in the skin and fi brous tissues, such as drying or coarsening of the skin, loss of elasticity, and premature skin aging (2). Moreover, prolonged exposure to UV radiation can also induce Address all correspondence to Christoph Gfeller at christoph.f.gfeller@gsk.com.
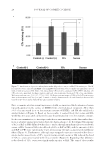
JOURNAL OF COSMETIC SCIENCE 2 permanent skin pigmentation, such as freckles and moles, and actinic keratosis, and may signifi cantly increase the risk of nonmelanoma skin cancer (NMSC) (2,3). Restricting exposure to the sun combined with regular use of sunscreen products with an appropriate sun protection factor (SPF) can limit the damaging effects of UV rays, includ- ing the risk of NMSC (1). SPF is an important measure of effi cacy for sunscreen products, providing an international standard for the level of protection provided against erythema induced by ultraviolet B (UVB) radiation (1). Sunscreen products containing active photoprotectant ingredients must undergo rigorous regulatory testing to ensure safety and effectiveness before approval is granted (4). This in- cludes in vivo UVB testing in accordance with either the U.S. Food and Drug Administration (FDA) monograph fi nal rule requirements (5) or international standard ISO 24444 (6). In addition to safety and effectiveness in the context of SPF, another important consider- ation for the development of new lip balm formulations is their moisturizing abilities, i.e., the reduction of water loss from the underlying tissue. The stratum corneum (SC), a lipid matrix in the outermost layer of the skin, is crucial for skin permeability barrier function and acts to prevent excessive water loss through the skin. One of the most important parameters for determining SC barrier function is transepidermal water loss (TEWL), the loss of water by passive evaporation through the skin (7–9). Separately, skin hydration can be measured using a variety of techniques, and instruments such as Skicon® (I.B.S. Co., Hamamatsu, Japan), DermaLab® (Cortex Technology, Hadsund, Denmark) Moisture Meter (DMM), and Corneometer® (Courage + Khazaka Electronic GmBH, Cologne, Germany) are considered the industry standards for this purpose (10). Newer lip balm formulations have been developed to not only provide protection from the sun, but also with the potential to hydrate dry lips. These new lip balm formulations include emollients and lipids combined with glycerin, and photoprotectant compounds that provide defi ned SPF. To meet regulatory requirements and obtain approval for use, rigorous clinical testing of these new lip balm formulations is required. Data from such clinical studies may also support product claims, including those pertaining to potential skin hydration and/or moisturizing abilities. Here we present data from fi ve clinical studies evaluating a series of novel lip balm formulations. The aim of four of these studies was to determine the SPF of fi ve new lip balm formulations to comply with sun protection labeling as defi ned by the U.S. FDA monograph fi nal rule (5) and international standard ISO 24444 (6). The aim of the fi fth study was to develop a new in vivo methodology to help differentiate lip balm formulations that have the potential to hydrate dry lips. In this study, the moisturizing abilities of one of the new lip balm formulations was fi rst compared with four comparator/reference lip products using a dry-skin leg model and evapo- rimeter (TEWL), Skicon, and Corneometer methodologies. Subsequently, an exploratory, home-use phase was conducted to assess the moisturizing abilities of this lip balm formulation over time using a novel dry-lip model and similar skin hydration assessment methodologies. MATERIALS AND METHODS STUDY DESIGN AND CONDUCT Barrier function and moisturizing abilities study. Study RH02116 was a randomized, six-cell, block design study to evaluate the effect on barrier function and moisturizing abilities of
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)