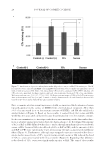
SACRAN PROTECTS SKIN AGAINST POLLUTANTS 29 demonstrates that sacran reduced the penetration of ACs and BaP in tobacco smoke and that the effect was higher than that of HA. To investigate the effects of sacran from the biological viewpoint, we examined the responses of HaCaT keratinocytes to tobacco smoke. In general, it is known that BaP upregulates CYP1A1 through the activation of AhR signaling. CYP1A1 metabolizes PAHs by intro- ducing a hydroxyl group to detoxify BaP. It has been reported that superoxide anion radicals are synthesized as a side product in the metabolic process (15–17). Thus, the ef- fects of sacran as a barrier against tobacco smoke were evaluated regarding CYP1A1 mRNA expression levels in HaCaT keratinocytes. Sacran and HA suppressed mRNA expression levels of CYP1A1 stimulated by tobacco smoke, and, furthermore, the suppres- sion by sacran was signifi cantly higher than that by HA (Figure 4). In addition, sacran ameliorated the cytotoxicity and elevations of intracellular ROS and intracellular CPs Figure 8. Interference with protein carbonylation in corneocytes exposed to tobacco smoke. Tape-stripped corneocytes were treated with sacran or HA, placed in a box fi lled with tobacco smoke for 2 h, and then were incubated for 24 h at 37°C. The levels of CPs in corneocytes was quantifi ed by image analysis. (A) Changes in protein carbonylation in corneocytes. Each value represents the mean ± SD of three independent experiments using the same fi ve volunteers. Wilcoxon rank-sum test, **p 0.01. (B) Representative images of CPs in corneocytes after each treatment (scale bar, 100 μm). Control(-) denotes sham-treated corneocytes and Control(+) denotes corneocytes treated with tobacco smoke without polysaccharide treatment.
JOURNAL OF COSMETIC SCIENCE 30 initiated by tobacco smoke (Figures 5–7). The effects of sacran also were higher than those of HA. The reduction in CP level was understood to originate from the improving effects of sacran on skin conditions from the aspect of oxidation because the long-term ap- plication of sacran reduced CP levels in corneocytes obtained from healthy volunteers (25). Sacran is a unique cyanobacteria-derived sulfated polysaccharide that is composed of 11 kinds of monosaccharides, which contain a sulfate group in 11% of them and a carboxyl group in 22% of them. However, the detailed structure of sacran regarding its amino acid sequence and amounts of lesser components has not been identifi ed. Considering the manner of the shielding effects indicates the possibility that polysaccharides trap BaP and/or ACs in their matrix. However, sacran and HA showed different intensities in their shielding effects, despite the difference in the presence or absence of sulfate groups in their chemical structures. Because carrageenan (a sulfated polysaccharide) did not show a shielding effect in a preliminary study (data not shown), it is hard to explain the differ- ence in the shielding effects between sacran and HA against BaP or ACs according to the presence or absence of sulfate groups. In our previous study, we found that sacran and HA showed different emulsifi cation abilities against squalene (data not shown). Sacran was able to emulsify squalene itself, but HA failed to emulsify squalene. Those results sug- gested the possibility of the presence of the hydrophobic domain to hold nonpolar chem- icals in the matrix formed by sacran. It might be considered that the hydrophobic domain in the matrix of polysaccharides depends on the difference of molecular weights. How- ever, the manner of the shielding effects of sacran is not beyond a region of hypothesis. Clarifying the mechanism involved in the shielding will require further study. CONCLUSION We conclude that sacran protects against adverse effects initiated by exposure to tobacco smoke, which is a representative air pollutant, because of shielding effects. These fi ndings strongly support the possibility that skin care products formulated on sacran will protect the skin against air pollutants. ACKNOWLEDGEMENTS We are grateful to Mr. Y. Waki, CEO of DAITO KASEI KOGYO CO., LTD., for his un- derstanding and cooperation on this research. REFERENCES (1) World Health Organization Publication, Ambient Air Pollution: A Global Assessment of Expo- sure and Burden of Disease, 2016, accessed April 25, 2018, http://www.who.int/iris/bitstream/ 10665/250141/1/9789241511353-eng.pdf?ua=1” title=”http://www.who.int/iris/bitstream/10665/ 250141/1/9789241511353-eng.pdf?ua=1” http://www.who.int/iris/bitstream/10665/ 250141/1/9789241511353-eng.pdf?ua=1. (2) R. Adelman, R. L. Saul, and B. N. Ames, Oxidative damage to DNA: relation to species metabolic rate and life span, Proc. Natl. Acad. Sci. U.S.A., 85, 2706–2708 (1988). (3) B. Karten, U. Beisiegel, G. Gercken, and A. Konstusk, Mechanism of lipid peroxidation in human blood plasma (a kinetic approach), Chem. Phys. Lipids, 88, 83–96 (1988). (4) E. R. Stadtman, Protein oxidation and aging, Science, 257, 1220–1224 (1992).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)