SURFACTANT PENETRATION INTO HUMAN SKIN AND RESULTING SKIN DRYNESS 37 Tape strip and cup scrub samples were analyzed for two individual surfactant components for each test formulation by stable isotope-based reversed-phase HPLC with tandem mass spectrometry using multiple reaction monitoring. Standard curves were constructed based on the peak area ratio of each analyte to the stable isotope internal standard versus the concentration of the standard. The concentration of the analyte in the cup scrub solu- tion was then determined by the peak area ratio of the sample by interpolation from the regression curve. Tape strip results were then pooled. 14 C-SODIUM DODECYL SULFATE (14C-SDS) SKIN PENETRATION EX VIVO This method closely follows that described by McCardy et al. (16). Split-thickness hu- man cadaver skin was obtained from the New York Firefi ghters Skin Bank (New York, NY) and stored at -80°C until use. Excised skin was cut into small pieces approxi- mately 1–1.5 cm2 in size and mounted in Franz diffusion cells (area = 0.79 cm2) with the stratum corneum facing up. Skin samples were allowed to equilibrate in phosphate- buffered saline (PBS Sigma Aldrich, St. Louis, MO) with 0.02% w/v sodium azide (NaN3 Fisher Scientifi c, Pittsburgh, PA) for 1–2 h and then integrity of the skin membranes was assessed by tritiated water (3H2O Perkin-Elmer, Waltham, MA) permeation using the Kasting et al. method (16). Skin samples with water permeation greater than 2 μL/cm2 were discarded. Test formulations were assigned to skin samples using a complete randomized block design with 3 H2O permeation as the blocking factor. Test formulations were prepared at 1.5% w/v total surfactant to simulate realistic cleans- ing exposures. Shampoos are typically formulated with approximately 15% w/v total surfactant, and we have estimated that consumers remove 90% of that material upon initial rinsing. Test formulations were spiked with 10 μCi/mL of radiolabeled 14 C-SDS (American Radiolabeled Chemicals, St. Louis, MO). On the morning of the study, recep- tor solutions were replaced with 4.25 mL of fresh PBS + 0.02% NaN3 and 150 μL of radiolabel-spiked test formulation was dosed into each donor chamber. After 2 min, ex- cess formulation was removed and collected. The surface of the skin was rinsed with three aliquots of 0.5 mL Millipore water by sequential up/down pipetting three times each, and these rinses were collected into one vial. The receptor solution was collected and then the skin sample was wiped once with Whatman fi lter paper (GE Healthcare Life Sciences, Issaquah, WA) soaked with PBS and then three times with Whatman fi lter paper soaked with 70% (w/w) ethanol to remove any residual test formulation. The fi rst three wipes were collected together and the last wipe was collected into a separate vial to ensure the radioactive test formulation had been suffi ciently removed from the skin surface. Skin samples were dissolved in 2 mL Solvable (Perkin-Elmer) at 50°–60°C overnight. Ultima Gold XR scintillation cocktail (Fisher Scientifi c) was added to each component, and these solutions were analyzed for radioactivity in disintegrations per minute using liquid scintil- lation counting via an LS 6500 Beckman counter (Beckman Instruments, Hebron, KY). Results were reported as percent of applied radioactive dose penetrated into the skin, including material that permeated through the skin into the receptor solution. A total of six skin donors were used. Each product was tested on two to three samples of each of fi ve skin donors, and four of the seven products (C, D, E, and G) were additionally tested on one sample of a sixth skin donor.
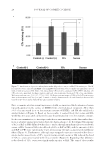
JOURNAL OF COSMETIC SCIENCE 38 DATA AND STATISTICAL ANALYSIS SAS (SAS, Cary, NC), JMP™ (SAS), and SigmaPlot (Systat Software, San Jose, CA) software were used for statistical analyses. A p-value 0.05 was used to determine statistical signifi - cance. The two clinical skin hydration measures (corneometer reading and visual dryness by expert grader) conducted in the FCAT were analyzed using a mixed model analysis of cova- riance. Analyses modeled subject as a random factor, and side, site, and treatment as fi xed factors. Baseline measurement and age were tested for signifi cant effects as covariates for both clinical measures. Based on these results, baseline measurement was used as a covariate for corneometer readings, whereas baseline measurement and age were used as covariates for visual dryness. Studentized residuals beyond four standard deviations were assessed as pos- sible outliers however, no outliers were removed in the fi nal analyses. Results are reported as adjusted mean change from baseline (CFB). Individual surfactant components extracted from either tape strips or cup scrubs in the FCAT study were calculated as masses extracted, and arithmetic means were reported. Ratios of masses extracted by tape strips to masses extracted by cup scrubs were calculated for each subject, and medians were reported. Re- sults from the ex vivo surfactant-skin penetration study were log10-transformed to achieve normality, and a two-way analysis of variance (ANOVA) with treatment and skin donor as the factors was used to obtain the least squares mean and standard error of the mean (SEM) for each test formulation. The geometric mean and standard error were calculated from these values and reported. Comparisons across the in vivo and ex vivo methods were com- pared using linear regression. Pairwise correlations using means were determined using Pearson’s Chi-squared test. RESULTS Results from the FCAT and 14 C-SDS skin penetration studies are provided in Table II. Clinical mildness is associated with higher corneometer readings (skin hydration) and lower visual dryness scores. The use of all test formulations led to signifi cant reductions in skin hydration and signifi cant increases in visual skin dryness versus baseline values. The mildest formulation (G) demonstrated a 5.2 decrease in corneometer reading with a 0.7 increase in dryness score, whereas the least mild formulation (A) demonstrated a 13.1 decrease in cor- neometer reading and a 1.5 increase in skin dryness score. Mean corneometer and visual dryness scores yielded similar rankings with respect to formulation-induced skin dryness. The clinical measures were found to correlate well with 14 C-SDS skin penetration ex vivo (corneometer reading, R2 = 0.75, p 0.05 visual dryness scores, R2 = 0.78, p 0.01). Comparisons of tape strip and cup scrub extractions are listed in Table III. We found the ratio of surfactant mass extracted by fi ve pooled tape strips to surfactant mass extracted by cup scrubs to be 40–59%. Quantities of individual surfactants extracted from fi ve pooled tape strips and cup scrubs are listed in Table II and illustrated in Figure 1. SLE1S penetration into the skin as measured by cup scrubs in vivo was found to correlate well with 14 C-SDS skin penetration ex vivo (R2 = 0.76, p 0.05) (Figure 2). Signifi cant and nearly signifi cant relationships emerged between CAPB penetration into the skin from cup scrub and tape strip in vivo and ex vivo skin penetration results when examining the anion-based test formulations only. CAPB penetration into the skin as measured by cup scrubs in vivo approached a signifi cant correlation with 14 C-SDS skin penetration ex vivo (R2 = 0.95, p = 0.15) for the anion-based systems (Figure 3). CAPB
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)