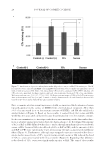
HYDRATION AND SPF TESTING OF LIP BALM FORMULATIONS 13 In study RH02116, three AEs were reported but each was considered by the investigator to be unrelated to study treatment. One AE was also a SAE (acute cholecystitis) and occurred during phase 1 of the study. The remaining two AEs (dysmenorrhea and upper respiratory tract infection) were both mild in severity and occurred during the home-use phase of the study. No AEs were reported in studies RH01928, RH02117, and RH02385. One AE (tingling sensation at the irradiation site of MED #1) was reported in study RH01927 but was not considered to be product related by the investigator because it occurred before product application. This participant continued in the study. There were no cases of prior or concomitant medication use, or concomitant nondrug treatment/procedures that were considered to have affected the study outcomes. DISCUSSION The dry-skin leg model used to help assess lip balm formulations in study RH02116 is a recognized industry method used for the evaluation of moisturizing products on body skin (11,12). Using this model, test product B demonstrated a statistically signifi cant improvement in barrier function and skin hydration across all postapplication time points compared with untreated dry leg skin. Furthermore, test product B signifi cantly improved skin surface hydration compared with four commercially available comparator products, and improved barrier function to a greater extent than three of the comparators. However, although these data are encouraging, they are based on a dry leg skin model. Lip skin differs from skin on the rest of the body in several ways, including the absence of sweat glands (and often sebaceous glands) and by having a thinner SC (18,19). As a result, our data derived from the dry leg skin model will need to be replicated using a dry lip model to determine the specifi c benefi ts of test product B for lip skin. Data from the exploratory novel dry lip model, derived from four different methodologies, produced variable results across all time points. This may be because of the small number of participants that were studied. In addition, the use of multiple methods in parallel to measure skin hydration may have made it more diffi cult to determine which data were the most relevant and reliable. As an example, the Corneometer method can be less sensi- tive to immediate changes in hydration of the SC a method that is sensitive to immediate changes in hydration of the SC is essential when evaluating lip balm formulations. There- fore, further investigation of current methodology is required. The four SPF determination studies were conducted in accordance with the requirements of FDA fi nal rule (5) and the international standard ISO 24444 (6). The mean SPF for the P2 positive control for all four studies was within the accepted range to meet the relevant regulatory criteria supporting the accuracy of the study protocols. However, although the early stage lip balm formulations tested demonstrated some SPF capability, results from the four SPF studies indicated that each of the formulations failed to meet the require- ments for the intended labeled SPF. Consequently, reformulation and retesting will be required. Nevertheless, and importantly, no product-related AEs or SAEs were observed for any of the lip balm formulations assessed. In study RH01927, test product A achieved a mean SPF value of 13.7 the SPF of this lip balm formulation would be labelled as 10 (low), according to FDA fi nal rule (5), which is lower than the intended SPF 15. In study RH01928, test product A achieved a mean
JOURNAL OF COSMETIC SCIENCE 14 SPF value of 15.2 according to ISO 24444 criteria (6). In study RH02117, the number of valid SPF results obtained was deemed insuffi cient, and it was therefore not possible to determine the SPF label for either test product B or test product C. In study RH02385, the mean SPF value for each of test product D and test product E was lower than the in- tended SPF 15. As a result, both lip balm formulations would have been labelled as low sun protection under the ISO 24444 criteria, and labelled SPF 12 and SPF 11, respectively, under the FDA criteria. A key strength of the SPF studies that we conducted is that the methodology we used to determine SPF follows industry standards and protocols as defi ned by the relevant regula- tors [FDA monograph fi nal rule and international standard ISO 24444 (5,6)]. CONCLUSION A new range of lip balm formulations was developed to provide sun protection and mois- turizing abilities. A dry-skin leg model was used to assess the potential moisturizing abilities of one of the new lip balm formulations. Using this model, the test product demonstrated improved leg skin hydration and barrier function properties compared with untreated dry skin, and com- pared with commercially available comparator products. Further testing is required to confi rm these benefi ts on dry lip skin. Data from an exploratory study that used a novel dry-lip hydration model, combining four different assessment methodologies, proved un- reliable. Therefore, further investigation of the lip-hydrating potential of the lip balm formulation is needed. The lip balm formulations studied herein were all rigorously tested to determine their SPF label according to protocols defi ned by relevant regulatory authorities [FDA monograph fi nal rule and international standard ISO 24444 (5,6)]. The SPF values of the tested lip balm formulations did not achieve the intended SPF levels. As such, they will undergo reformulation and retesting to satisfy the required SPF levels. ACKNOWLEDGMENTS The authors would like to thank the participants in these fi ve studies. Editorial support in the form of the development of a draft manuscript in consultation with the authors, and preparation of tables and fi gures was provided by Victoria Pugh, PhD, of Gardiner-Caldwell Communications, Macclesfi eld, UK. FUNDING These studies were funded by GSK Consumer Healthcare (GSK studies RH01927, RH01928, RH02116, RH02117, and RH02385). DISCLOSURES C. Gfeller, G. Hardie, G. Shanga, and H. Mahalingam are all employees of GSK Consumer Healthcare. No additional disclosures to declare.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)