798 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS However, among many important factors such as mixing and cooling rates which are known to affect emulsions, one factor which has not been thorough- ly studied is the location of the surfactant in the emulsion (2). In manufacturing emulsions, it is generally regarded best to form th• emul- sifier in situ (3). However, except when fatty acid soaps are used as the emul- sifters, it is usually impractical to prepare emulsions by such a method. More frequently, the surfactant is either dispersed in the oil or aqueous phase prior to emulsification. Since all surfactants have some solubility in both oil and wa- ter, if the surfactant is first dispersed in the oil phase containing all oil-soluble components and then added to the aqueous phase to form an emulsion, some of the surfactant originally in the oil phase will migrate to the aqueous phase until an equilibrium is established. Conversely, migration would take place from the aqueous phase to the oil phase if the surfactant were first placed in the aqueous phase. The main purpose of this work was to determine if the initial surfactant lo- cation and the migration of the surfactant immediately after emulsification had any significant effect on the stability and droplet size distribution of the emulsion prepared. Theoretically, since the surfact.ant plays a major role in stabilizing the emul- sion, any movement of the surfactant during the emulsification process can affect the adsorption of the surfactant at the interface and thus influence the quality of the emulsion formed. If the equipment or procedure used for emul- sification affects surfactant migration, the formation of the emulsion may also be indirectly affected. Similarly, the migration of the surfactant after the for- mation of an emulsion can also alter the emulsion properties and emulsion stability. Conceivably, this may be one of the factors controlling the often troublesome changes in the rheological properties of freshly prepared cosmet- ic emulsions upon aging. However, in order to quantitatively study the effect of surfactant migration on emulsion stability, one must accurately know the distribution of the surfac- rant in both phases of the emulsion at a given time. There is definitely a paucity of such data as the measurement of surfactant distribution in an emul- sion is no simple task. This is because, first of all, the surfactant is not only dis- solved in the bulk phases of the emulsion but is also present as micelles in both phases and a substantial portion of it is adsorbed at the oil/water interface of the emulsion droplets. Secondly, no method is available which allows a direct measurement of the surfactant concentration in either the continuous or dis- persed phase of a stable emulsion without first breaking or creaming the emul- sion. If an emulsion is first cracked by coalescing the dispersed droplets using chemical or physical means, the surfactant present in one of the phases or in both phases can then be analyzed without too much difficulty. However, this would only provide the information on the surfactant distribution in a cracked emulsion. In order to know the distribution in the original stable emulsion,
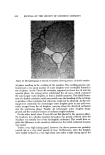
EMULSION STABILITY 799 one would have to know the migration of the portion of the surfactant de- sorbed from the interface when the coalescence took place. Clearly, it would be extremely difficult to experimentally or theoretically follow precisely the complex movements of the surfactant molecules after desorption. Thus, the major difficulty in measuring the surfactant distribution in a stable emulsion is that the means of the measurement would invariably cl•.ange the original distribution, making the interpretation of the results extremely diffi- cult. T.o overcome this difficulty, a method was devised to allow a gradual cracking of an emulsion so as to permit an indirect measurement of the origi- nal distribution from the data obtained at different stages. This was accom- plished by a successive centrifuge of the stable test emulsion, followed by a chemical analysis of the surfactant content in the separated phase at each stage, and, finally, by the extrapolation of the data to zero separation. By mak- ing such measurements on emulsions prepared with different oils at different intervals after emulsification, and also by measuring the particle size distribu- tion of emulsion droplets by microphotography, the effect of surfactant migra- tion on the emulsion stability was investigated. EXPERIMENTAL Emulsions were made both under a very high mixing speed using T-K H.omomixer Model M* and also under a relatively low mixing speed using a paddle mixer. For rapid mixing emulsification, 2-kg batches of emulsions were made in 3-liter beakers using the following formulation: % by Wt. Oil 32.00 Arlacel 804•i 1.40 Tween 80©i 1.60 Carbopol 934©$ 0.10 NaOH 0.04 Deionized water 64.86 100.00 In this work, only the migration of the hydrophilic surfactant, Tween 80, was followed. The hydrophobic surfactant, Arlacel 80, which has a very low water solubility, was dispersed in the oil phase before emulsification. The aqueous phase consisted of water and Carbopol 934 neutralized with sodium hydroxide. The migrating surfactant, Tween 80, was divided between the * Manufactured by Tokushukita Kogyo Co., Ltd., Osaka, Japan. •' Ariaeel 80 (Sorbitan monooleate), Tween 80 (Polyoxyethylene sorbitan monooleate), Atlas Chemical Industries, Wilmington, Del. $ Carbopol 934 (Carboxyl vinyl polymer), Goodrich Chemical Co., Cleveland, Ohio.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)