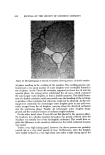
812 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Figure 15. Microphotograph of mineral oil emulsion showing presence of double emulsion oil phase resulting in the swelling of the micelies. The swelling process con- tinued and a very great number of water droplets were eventually formed in the oil phase. As the Tween 80 molecules migrated out from the oil into the aqueous phase, the mixing action subdivided the oil mass, which contained the microscopic •vater droplets, to form a double emulsion. The initial forma- tion of a double emulsion could conceivably ease the breaking of the oil drops to produce a finer emulsion that otherwise could not be obtained. As the mix- ing process continued, the microscopic water droplets grew in size and even- tually escaped from the oil droplets, carrying along the dissolved surfactant into the conth•uous phase. Finally, all microscopic water droplets disap- peared and the emulsion became an ordinary single emulsion. On the other hand, when T•veen 80 was first placed in the aqueous phase, the tendency for a double emulsion formation was greatly reduced since the oil phase was initially free of the hydrophilic surfactant. This would then ex- plah• the difference in the emulsions obtained as the initial surfactant location was varied. Under a very high mixing speed, ho•vever, the above process was probably carried out in a very short period of ti•ne. Furthermore, since the droplets were readily broken by a very high shear rate under a high mixing speed, the
EMULSION STABILITY $13 initial formation of a double emulsion was probably no longer an important factor controlling the droplet size of the final emulsion. CONCLUSIONS In this work, the suroeactant migration was monitored only in the emul- sions prepared under very rapid mixing using a Homomixer. Unoeortunately, it was not possible to measure the suroeactant distribution accurately in emul- sions prepared under low mixing speeds as these emulsions were too unstable when subjected to centrifuge. It is hoped that the present technique can be improved in the future to allow handling of emulsions prepared under lower mixing speeds. On the other hand, photographing of the emulsification pro- cess was possible only for the emulsions prepared under very gentle mixing conditions. Most commercial cosinefie emulsions are prepared under neither an ex- tremely high mixing speed, nor a very low speed. Moreover, only liquid oils were used as the internal phase in this work, whereas in commercial cos- metics, various waxes and thickeners are used along with the oils. Hence, the actual migration of the surfactant in a commercial emulsion may be consider- ably slower than that which took place in the systems studied here. Due to the complex nature of the mechanisms goveming the emulsion formation and surfactant migration, the results obtained here cannot be directly ap- plied to commercial emulsions without qualification. However, it seems safe to make a few limited generalizations based on the data obtained. 1. In preparing emulsions, suffcient mixing should be provided not only to reduce the droplet size but also to promote surfactants to attain an equilib- rium. 2. If the cquilibrium is not attained upon completion of emulsification op- eration, the surfactant migration thereafter may cause a change in the physi- cal properties or the stability of the emulsion. 3. If an O/W emulsion is made under a moderate mixing speed, it is prob- ably better to place the surfactant initially in the oil phase in order to obtain an emulsion with a finer particle size distribution. However, as pointed out by Lin and Lambrechts, if the HLB of the surfactant mixture is low, this practice may produce a phase inversion which may, in turn, cause emulsion instability (5). Therefore, in making a practical emulsion, the best surfactant location should be decided by carefully controlled experiments. ACKNOWLEDGMENT The authors gratefully acknowledge the encouragement and valuable sug- gestions given by Dr. T. Moroe and Dr. A. Komatsu and also the experimental assistance of Mr. M. Nakatomi of Takasago Perfumery Co., Ltd. (Received December 7, 1972)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)