810 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table I Stability Difference between Emulsions Prepared with Tween 80 Initially in Oil (Emul- sion O) and Corresponding Emulsions with Tween 80 in Water (Emulsion W) for Various Oils (Slow Mixing) Emulsion O considerably better than Emulsion W Emulsion O slightly better than Emulsion W No significant difference Oleyl alcohol, n-decyl alcohol, 2-octyl- dodecyl alcohol, oleic acid, ricinoleic acid, linoleic acid, isopropyl myristate, dioctyl phthalate, dicthyl phthalate, diethyl sebacate, methyl benzoate, hexadecyl lanolate, octyldecyl trigly- ceride, cottonseed oil, rapeseed oil 2-tlcxyldecyl alcohol, liquid lanolin, di~ 2-hexyldecyl ether, 2-hexyldecyloctyl ethcr, mineral oil (70 cps), mineral oil (350 cps), squalan, castor oil, olive oil, soybean oil Isostearic acid 3O • 20 •1o 0 20 40 60 80 100 % TWEEN 80 IN AQUEOUS PHASE Figure 13. Effect of mixing speed and initial surfactant location on stability of oleyl alcohol emulsions (after 5-rain mixing and 3-rain standing)
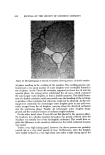
EMULSION STABILITY 811 Figure 14. Microphotographs of oleyl alcohol emulsions Top. Surfactant initially in oil Left to right. Emulsions O-1, 0-2, 0-3 Bottom. Suffactant initially in water Left to right. Emulsions W-l, W-2, W-3 at various stages of emulsification. Some of the examples are shown in Fig. 14. Examination of these microphotographs indicates the presence of a large number of double emulsions of (W/O)/W type in the emulsions prepared by initially placing the surfactant in the oil phase (i.e., Emulsion O series). As shown in the photograph O-1, which was taken at an early stage of emulsi- fication, extremely small water droplets were observed in very large oil drops. Sometimes the boundaries of these oil drops were not well-defined at this stage. The picture O-2 clearly indicates the presence of double emulsion droplets. These double emulsion droplets disappeared after a prolonged mix- ing as shown in 0-3. Double emulsions were also observed in some emulsions prepared by initially placing the surfactant in the aqueous phase (i.e., Emul- sion W series) but the number of droplets containing the double emulsion was much lower. A close examination of the microphotographs suggests that the formation of the double emulsion might be the reason for the formation of fine emulsion even under very slow mixing when the surfactant was placed in the oil phase. An enlarged photograph of such a double emulsion is shown in Fig. 15. One possible mechanism is that when the emulsion was made by first placing Tween 80 in the oil phase, water initially entered the surfactant micelies in the
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)