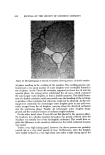
822 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS IO0 F Mean and Range Mean and Range for 68 runs for 58 runs SnF2 SOLUTIONS• t. .001 .005 .01 .02 o NaF SO L UTION.•._.•.•,_.._.• I I I .05 .04 05 CONCENTRATION of F7 M Figure 4. Dissolution curves oE intact human dental enamel treated with sodium and stan- nous fluoride solutions [from (9)] Certainly if clinical studies are required to verify laboratory documented im- provements of the basic dentifrice system, few if any of these improvements will be made or utilized. Because of the widespread availability of anticaries agents, it is becoming increasingly difficult to conduct a clinical study. It will become more difficult to establish clinical anticaries activity. For this reason, continued emphasis must be placed on the design of the clinical study to maximize the information received and clinical studies should be undertaken only when the need is in- dicated. There must be greater reliance upon definitive laboratory studies. The basis for this greater reliance upon laboratory studies is the correlation of experimental data of the new product with the clinically established prod- uct. Comparison with activity of the stannous fluoride dentifrices seems in- dicated. LABORATORY EVALUATION Laboratory data on sodium fluoride and monofluorophosphate are less ex- tensive than those for stannous fluoride and probably less impressive. Since these compounds have been demonstrated to be clinically effective (5) with an activity somewhat comparable to stannous fluoride dentifrices, then new laboratory procedures must be developed to assess this activity. The following methods have been useful in characterizing dentifrice formu- lations containing stannous fluoride. The enamel solubility test is a good meas-
THERAPEUTIC DENTIFRICES 823 ure of the bioavailabilty of stannous fluorde because of the correlations with clinical and other studies using different types and ages of stannous fluoride formulations (23). Methods Chemical A,mlysis (24) A slurry is prepared of 10 g of paste and 30 ml of water in a 50-ml centrifuge tube using a stirring rod fitted with polyethylene tubing cut in strips to facili- tate mixing. Stirring follows for 2 min or until a uniform slurry is obtained. The tube is capped and centrifuged at 15,000 rpm for 5 min (total time about 15 min.) to obtain a clear supernatant. For fluoride ion analysis (6, 13), an accurately measured aliquot of the supernatant or dilution thereof equivalent to about 1-2 /xg of fluoride is transferred to a suitable diffusion vessel (25, 26) and the fluoride ion is sepa- rated from the interfering ions. The diffused fluoride is collected and deter- mined colorimetrically using the SPADNS reagent or fluoride electrode. Total fluoride concentration can be determined by fixing the paste with alkali, ash- ing, and determining the fluoride by diffusion-colorimetric or electrode pro- cedure (24). For stannous ion analysis (7), an aliquot of the supernatant equivalent to about 3 mg of stannous ion is transferred to a titration assembly and is titrated potentiometrically using a platinum combination electrode and 0.005N iodate reagent. Total stannous and tin concentrations in paste can be determined by dissolving the paste in 6N hydrochloric .acid and fitrating a diluted aliquot with iodate reagent (24). Enamel Solubility Reduction In general, it is convenient to run enamel solubility tests in groups of four. Twenty-four caries-free teeth which have a minimum number of defects are selected. Teeth with larger enamel surface areas are preferred. These are pre- pared for mounting by scaling the enamel surface with a suitable dental in- strument and pumicing lightly using the rubber cup procedure. Preparation of Tooth Mounts (Fig. 5) Dental stone is mixed and transferred with gentle tapping to remove air bubbles to an aluminum pan (55 mm in diameter x 17 mm deep*) to a depth of about 12 min. The bottom and sides of a 50-ml beaker (o.d. 42 mm)* are coated with petrolatum and forced into the dental stone to a depth of about 10 mm. After the dental stone has reached a final set, the beaker is removed. *S•5725, Sargent-Welch Scientific Co., Skokie, Ill. 60076. ?No. 1000, Corning Glass Works, Corning, N.Y. 14830.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)