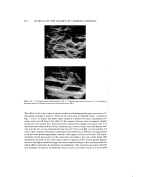
308 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS lution of glycine. From the rheological properties, the previously mentioned results can also be explained. In this case, a maximum of 19 times the amount of glycine aqueous solution by weight to the surfactant was contained as the inner phase. When a 40 per cent aqueous solution of monosodium L-glutamate monohydrate was used, a maximum of 46 times was obtained. A gel in the metastable state lasting only for several hours was obtained in the absence of an amino acid as in the case of a system containing only surfactant and water. The water particles in the gel soon coalesced and the gel finally separated into 2 phases. The changes of water particles with time in these two cases are compared in Fig. 6. It can be seen that the gels obtained by the addition of amino acids were very stable with no coalescence of droplets. The effect of pH on the gels was also examined. Figure 7 shows the stability of the gels obtained from buffer solution and that with a fixed amount of glycine (neutral amino acid, pI equals 5.97) L-arginine (basic amino acid, pI equals 11.15), L-glutamic acid (acidic amino acid, pI equals 3.22). It was ap- parent from the diagrams, that in all of cases, the gels were not stable in a higher pH range, but stable at the lower pH, i.e., the acid side. Gelation was poor in the range where the pH was extremely low and became better on the weakly acid side at 4.0 to 6.5. Stability also depended on the storage temperature. As the temperature increased, the stability shifted to the lower pH range. As will be described later, the hydration power of the amino acids is considered to relate to the formation of stable gel, and it may be determined depending upon the ionic state of the amino acids. From the fact that the aqueous solution of the amino acids and the above mentioned surfactants are combined to form gels having very high viscosity and showing no co- alescence of particles of the aqueous solution, it is presumed that some specific interac- tion exists between the amino acid and surfactant at the interface. In order to confirm the possible existence of this interaction, first, the structure of the gel was investigated by the measurement of the heat of solution, X-ray analysis, DTA, and microscopy. Figure 8 shows the results of the heat of solution at 35øC when the surfactant, Sunsoft O-30B, was mixed and dissolved into an aqueous solution of the amino acids. In the case of water only, exothermic heat of about 5 cal/g was measured. Should it be assumed that a new complex is formed by the addition of amino acid, an increase of exothermic heat higher than in the case of water will be expected and the curve should go upward. However, as shown in the figure, in both the case of L-serine and monosodium L-glutamate monohydrate, the heat of solution decreased rapidly even with a minor increase of concentration. This indicates that the solubility of surfactant to amino acid solution decreases more than that to water by the possible reduction of the interaction between amino acid and surfactant and also confirms the fact that there'are no new complex formations taking place. Subsequently, as a result of X-ray analysis of the gels, which is the most common method, two diffraction lines were found in the small angle. It corresponded com- pletely with the X-ray diffraction pattern of the surfactant itself as is previously described and was finally confirmed as that of the lameliar structure of the surfactants per se. However, it could not be confirmed that the formation of a new structure having new spacings between amino acid and surfactant was formed. The same results were
WATER-IN-OIL EMULSIONS 309 obtained by DTA and optical microscopic observations. From Fig. 6, it can be said that the gel itself is considered to be the same emulsion system as an ordinary w/o emulsion in which the particles of aqueous solution are dispersed in the lipophilic surfactant phase. It can be readily understood, as is shown in Fig. 5, that fluidity, high viscosity, and hardness of the gel are due to the increasing volume ratio and interfacial area. In the gels obtained by the addition of aqueous solution of amino acids, the interfacial films are very strong and stable so that the particles do not coalesce even though the in- ner phase is increased. These phenomena owe much to the functions of the amino acid. From these facts, the authors studied the influence of the amino acids on the change of the lameliar structure possessed in common with the surfactants used. Figure 9 indicates the comparison between the spacings of stable gels with aqueous so- lutions of amino acid and those of unstable gels with water only and urea. Sunsoft O-30B, which was used as the surfactant in the experiments, had spacings of 33.8 ,•. In the case of an aqueous solution of amino acid, even though the mixing ratio of the gel was altered, the original spacing of the surfactant did not change. However, in the case of water, it increased to 37.6 •. Such change had been determined in either case at an early stage, regardless of the increased mixing ratio. It may be considered that the " amino acid results in weakening the interaction between the water and the surfactant : that is, the amino acid decreases the solubility of the surfactant in the water phase and also prevents the solubilization of water into the hydrophilic parts of the lameliar struc- ture, therefore, it seems to prevent the change in the original structure of the ::"" surfactant. It was found that urea further increased the spacing than in the case of :. water. Urea formed a gel with difficulty and though formed, was immediately destroyed and inverted to the o/w type. The explanation of the results of NMR measurement are shown in Fig. 10 and shows how the amino acids or their salts interact with water. A negative sign Ad indicates a • shift of proton in water to the lower magnetic field, and a positive sign means a shift to the higher magnetic field. The amino acids allows the proton in water to shift to the ß :: lower magnetic field. The gradient was sharp, hence, the effect was much greater even in lower concentration. On the contrary, potassium thiocyanate (KSCN) and urea :- •: shifted the proton to the higher magnetic field. In other words, these facts suggest a change in the physical properties of water. An amino acid, which shifts the proton to the lower magnetic field, indicates a condition in which the affinity of water to the amino acid is very strong and results in a decrease of free water. Therefore, these •:i amino acids decreased the solubility of surfactant, which previously existed in water or prevented the solubilization of water in the hydrophilic part of the surfactant. These concepts are supported by the data obtained in the heat of solution shown in Fig. 8. Furthermore, since the amino acid will expel the surfactant from the water phase, it be- comes evident that a w/o type emulsion is the easier one to obtain. It is presumed that there is a decrease in the PIT with the increase concentration of the amino acids. This hypothesis was confirmed by the results of the PIT as is shown in Fig. 11. The amino acids decreased the PIT, and conversely, KSCN and urea increased the PIT. Such a trend in the PIT corresponded perfectly with the results of the NMR analysis. From this view point, it can be readily understood how the amino acid changes the physical properties of water and decreases the interaction with the surfactant. In order to inves- tigate how much water causes a change of structure when solubilized in the hydrophilic part of the surfactant, the authors compared the changes in the amount of water migrat-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)